ABSTRACT
Objective: To critically analyze the evidence regarding changes in verbal and performance intelligence quotient (IQ) in patients with schizophrenia.
Data Sources: An English-language–only search was conducted in the PubMed, Cochrane Library, and LILACS databases for articles with study objectives that included Wechsler Adult Intelligence Scale (WAIS) assessment of cognitive functions in patients with schizophrenia. Descriptors were defined based on Medical Subject Headings, where associations of psychotic disorders related to the schizophrenia spectrum were suggested, as well as the “Wechsler Scales” descriptor. The search was conducted in November 2022 with no restriction on the date of publication to select studies that used any of the WAIS editions.
Study Selection: Articles that met the inclusion criteria were selected after title and summary identification and full-text review.
Results: A total of 28 articles were identified. All studies presented total IQ scores, but only 20 showed results for verbal IQ (n = 20) or performance IQ (n = 19). Analyzed data indicated patients had average performance on verbal comprehension features but low average performance on perceptual reasoning, working memory, and processing speed indices.
Conclusions: Executive function deficits were found in the analyzed studies, which reflect difficulties in planning and impulse control—characteristics present in the diagnosis of schizophrenia. The identification of this neuropsychological functioning contributes to the understanding of the cognitive dynamics found in schizophrenia and may help in early diagnosis, reinforcing the hypothesis that cognitive performance may be one of the indicators of psychopathologic expression.
Prim Care Companion CNS Disord 2023;25(5):22r03456
Author affiliations are listed at the end of this article.
Schizophrenia is a severe mental disorder characterized by positive symptoms (eg, delusions, hallucinations, disorganized thinking, and abnormal behavior) and negative symptoms (eg, blunted affect, reduced speech, and disturbance of volition). These symptoms, according to the DSM-5,1 must be present for at least 6 months, in addition to having a disturbance in level of functioning in ≥ 1 major area of life, such as work, social relations, and self-care. Schizophrenia commonly begins to exhibit its signs and symptoms in late adolescence and early adulthood (around age 30 years), with a slightly higher incidence in men than in women.1
Understanding the etiology of schizophrenia begins with a multifactorial model for determining the disease, in which social, environmental, and biological factors are interrelated. The literature also notes the influence of regional and ethnic changes, as well as the experiences of adverse social situations throughout life.2 A study by Ruby et al3 found that social experiences and environmental aspects may effect epigenetic change to hereditary genetic features. Neuroscience suggests that environmental influences on gene expression may have important impacts on physiology, behavior, and disease development. Therefore, the severity of symptoms, as well as cognitive changes present in schizophrenia, may be determined by environmental stimuli, hereditary and physiologic aspects, and specific characteristics (eg, culture, age, sex).
Multiple deficits in cognitive functions can be present in people with schizophrenia, leading to significant effects on the individual’s functionality and social involvement.4 A literature review gathered data from 13 studies to identify the main cognitive changes present in schizophrenia.5 The authors5 concluded that 7 specific cognitive factors were repeated among these studies and thus represented fundamental dimensions of this population’s cognitive deficits: processing speed, attention, working memory, verbal memory, visual memory, logical reasoning, and problem solving. Nevertheless, Callicott et al6 noted, in functional magnetic resonance imaging and memory tasks, that the alterations in neural circuitry of the prefrontal cortex regions interfered in planning and attention skills in a sample of 9 individuals with schizophrenia, which are reflected primarily as working memory impairment. Yet, the authors6 argued that impairments in social judgment and decision-making ability produce maladaptive behavior, as they are directly related to the ability to process information and manipulate it to solve problems. The results of these studies are in agreement with Heinrichs,7 who indicated that cognitive functioning is a more significant marker than psychiatric symptoms and thus can compromise functionality in individuals with schizophrenia.
When compared to healthy individuals (n = 176), the study by Kudo et al8 showed that those with schizophrenia (n = 87) scored lower on the Wechsler Adult Intelligence Scale (WAIS-III) in total intelligence quotient (IQ) and scored below average in complementary indices (perceptual organization, working memory, and processing speed). In another study, Lin et al9 assessed 1,200 individuals with schizophrenia and 76 healthy controls regarding sustained attention (continuous performance task), executive function (Wisconsin Card Sorting Test), and the general intelligence scale (WAIS-III). This research showed a statistically significant difference between groups in cognitive functions that involve memory, attention, executive function, and processing speed.
Many studies have found cognitive deficits in patients with schizophrenia, particularly related to frontal functions, executive domains, attention, and visuospatial skills. However, little is known about the relationship between verbal and performance IQ and clinical variables of the disease such as age, intensity of symptoms, and duration of illness. Verbal IQ can be conceptualized as the ability to manipulate abstract symbols, comprehension, and verbal fluency, while the ability to solve problems, integrate perceptual stimuli and motor responses, and evaluate visuospatial information is described as performance IQ.10
The main objective of this review is to critically analyze the evidence regarding changes in verbal and performance IQ in patients with schizophrenia. Our hypothesis is that deficits in performance IQ, emphasizing attention, working memory, and perceptual organization, correlate with the difficulty of adapting to the social context, inability to follow important instructions, and impairment in the ability to plan and in impulse control, which are major clinical features of schizophrenia.
Although studies corroborate that there are deficits in cognitive functions of people with schizophrenia and that these functions differ between healthy control groups, sampling characteristics (eg, comorbidities, duration of illness, severity of symptoms, and use of medications that can influence cognitive performance) and the use of different assessment tools complicate efforts at meta-analysis and data comparison of cognitive function measures. Even if the same construct and theories underlie the various instruments with high psychometric quality, there may still be other differences in the results due to the sampling and methodological approaches used in each study.
Although there are various psychological tests for the assessment of cognitive functions, Michel et al11 noted that the WAIS is considered the gold standard in overall neuropsychological assessment. The WAIS is a broad test of general intellectual capacity, comprised of scales that cover 4 domains of cognitive functioning: verbal comprehension, perceptual organization, working memory, and processing speed.12 As a methodology, Wechsler12 adopted the evidence of individual WAIS differences as a starting point for solving different kinds of problems, as diverse mental functions are influenced by global intelligence. Intelligence levels for healthy individuals are classified by the Wechsler scales according to the IQ results obtained.
The objective of this systematic review was to identify characteristics of cognitive functioning in adults with schizophrenia on the WAIS tasks and determine what cognitive functions seem to be impaired in this population compared to scale results from a normative sample.
METHODS
An English-language–only search was conducted in the PubMed, Cochrane Library, and LILACS databases for articles with study objectives that included WAIS assessment of cognitive functions in patients with schizophrenia. These databases were chosen because they reflect a large portion of relevant international scientific data, thus meeting the criteria of comprehensiveness and representativeness.
The descriptors used in the search were “Schizophrenia OR Schizophrenia Spectrum and Other Psychotic Disorders OR Schizophrenia, Paranoid OR Schizophrenia, Disorganized OR Schizotypal Personality Disorder OR Schizophrenia, Catatonic NOT Schizophrenia, Childhood NOT DISC2 gene product, human NOT DISC1 protein, human AND Wechsler Intelligence Scales OR Intelligence Scales, Wechsler OR Wechsler Adult Intelligence Scale-Revised OR WAIS-R NOT WMS-IV-NL NOT OR Wechsler Preschool and Primary Scale of Intelligence NOT WPPSI NOT Wechsler Test of Adult Reading NOT National Adult Reading Test NOT Wechsler Intelligence Scale for Children NOT WISC-V OR WISC-IV.” Descriptors were defined based on Medical Subject Headings, when application to psychotic disorders in the schizophrenia spectrum are suggested, as well as the “Wechsler Scales” descriptor to sum the latest versions of the WAIS, WAIS-R, WAIS-III, and WAIS-IV. The search was conducted in November 2022 with no restriction on the date of publication to select studies that used any of the WAIS editions. Inclusion criteria for articles were (1) sample comprised of people diagnosed with schizophrenia aged between 16 and 84 years, (2) study aimed at assessing cognitive performance among the participants, (3) use of one of the editions of the WAIS as the main instrument for cognitive assessment, (4) English materials, and (5) correlational, descriptive, or clinical studies. Articles were excluded when the (1) sample was not characterized exclusively by adults with schizophrenia, (2) sample included participants with neurologic comorbidities, (3) authors did not identify the WAIS total IQ score, and (4) studies were described as case studies or bibliographic reviews.
After inserting the descriptors in the selected databases, manual screening of articles that met the study inclusion criteria was conducted. Those that met the inclusion criteria were selected after titles and summaries were identified and articles were read in full.
RESULTS
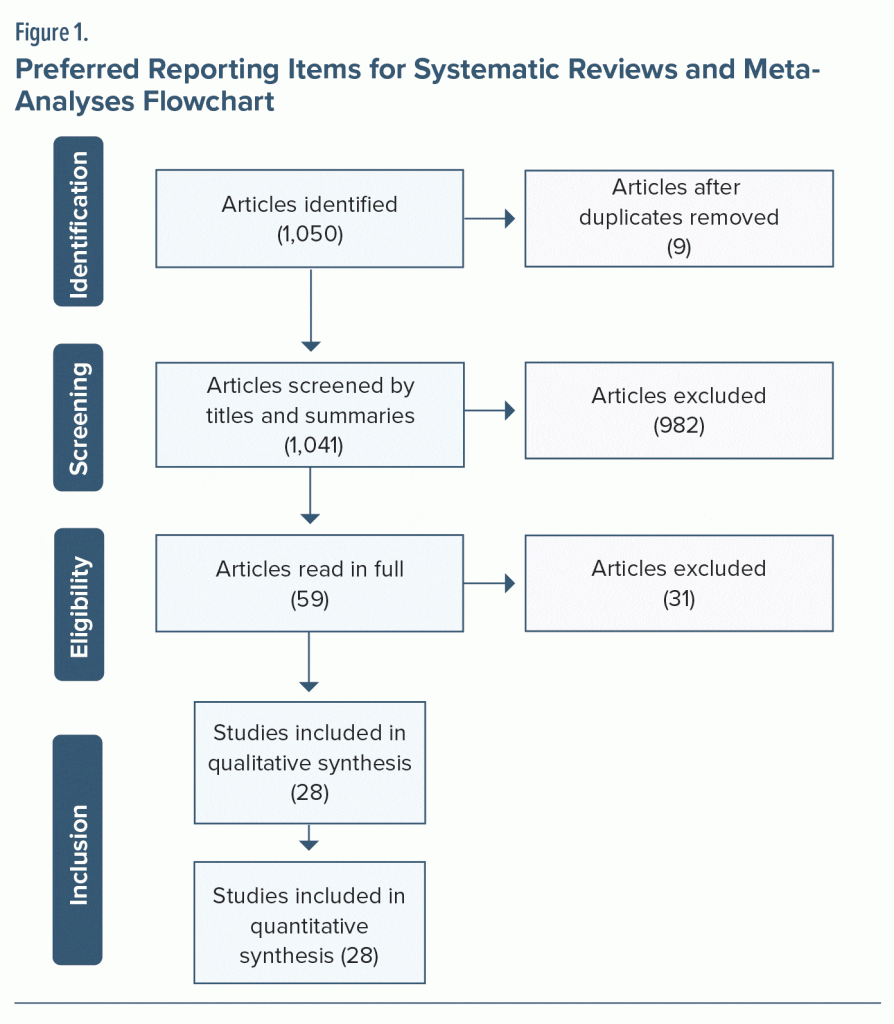
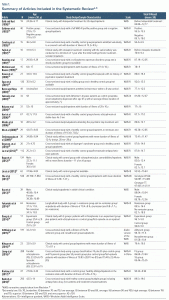
Twenty-eight articles in which participants were assessed using the WAIS were selected for analysis.8,9,13–38 The PRISMA flowchart is provided in Figure 1.39 Table 1 shows sample characteristics, study design, WAIS version used, and mean total IQ score and standard deviation. Studies included in the systematic review were published between 1990 and 2018, with a total sample size of 2,203 assessed subjects. The mean age varied between 22 and 64 years. Regarding study design, cross-sectional studies (19), clinical studies (8), and 1 longitudinal study were identified. As for the scale edition used, 4 studies used the first version (WAIS) of the instrument in their sample, 19 studies used the revised version (WAIS-R), 5 used the third edition (WAIS-III), and no study included in this review used the WAIS-IV, which is the current version of the scale published in 2008 (Table 1).
In 8 of the total articles selected, cognitive assessment was carried out with 2 sample groups of patients with schizophrenia. Thus, IQ results of 36 samples of individuals with a psychiatric diagnosis of schizophrenia were analyzed in this systematic review. In clinical research with medication administration throughout the study, results of the assessments made initially were analyzed without the interference of treatment on the sample’s cognitive performance.
All articles included in this systematic review described the mean and standard deviation of the total IQ result obtained by the sample assessed. Combined verbal and performance scales are transformed into a composite score according to methods defined in the instrument manual and may vary depending on the WAIS edition used.
For most samples (19 samples), the total IQ score was between 80 and 90 (low average), with 9 samples showing average total IQ scores and 6 samples showing extremely low total IQ scores. Considering the total of 36 samples, the calculated average corresponds to a value of 87.39, which is classified as low average in relation to the expected performance for the population studied during standardization and validation of the instrument.
The lowest average total IQ was 73.41 (SD: 11.47) in a sample of 36 subjects.27 The highest average total IQ was 98.73 (SD: 11.6), wherein 47% of subjects completed college, in addition to having an exclusion criterion for people with a total IQ index < 90.35
Comparison Between Verbal IQ and Performance IQ
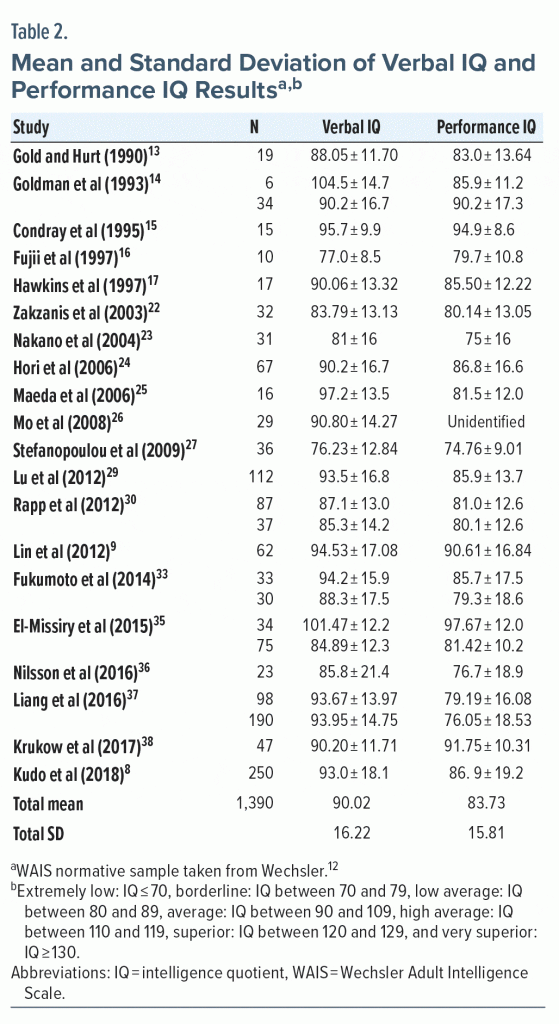
Only 20 studies showed the average verbal IQ performance, while 19 showed the average performance IQ (Table 2). The verbal intelligence quotient (VIQ) is defined based on performance in verbal scale subtests, which change according to the scale version used. Tasks included in this battery assess verbal comprehension, acquired knowledge, quality of formal education, and environmental stimuli. For this step, the ability to deal with abstract symbols is required, where information about language processing, reasoning, attention, verbal learning, memory, and verbal fluency is collected from the subject. Verbal assessment shows auditory and oral intelligence, which aids in expression and verbal conceptualization.14
VIQ performance was described in 20 articles, comprising 25 samples assessed, so that most of the samples (15) described the performance as being classified as average in comparison to that expected for the normative sample of healthy individuals. For some samples, performance was classified as low average (8 samples) and borderline (2 samples). VIQ indices had variations in the samples studied; however, the lowest VIQ performance mean (76.23 ± 12.84) is related to a sample of 36 participants,27 and the highest VIQ mean (104.5 ± 14.7) is from a sample of 6 individuals14 (Table 2).
Subtests comprising the performance intelligence quotient (PIQ) encompass cognitive domains related to the degree and quality of nonverbal contact, as well as their ability to integrate perceptual stimuli and produce pertinent motor responses, to work in concrete situations, and to assess visuospatial information. The performance aspect of the intelligence scale assesses the quality of sequential reasoning and the ability to establish relationships between events, analyzing the spatial component of perception at a conceptual and executive level, in addition to organizational and psychomotor performance. The performance scale is less influenced by formal education, as above-average results are usually associated with a high level of organizational skills and ability to work under time pressure.
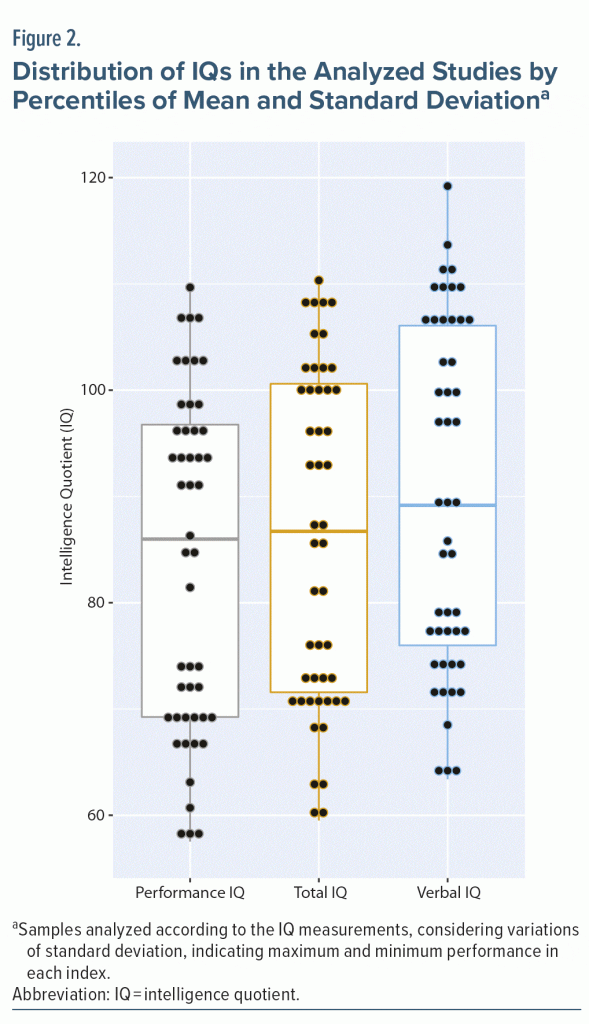
In this review, performance means of 24 samples with PIQ results were assessed. Most of these samples indicated low average performance (12 studies), and the other samples showed performance classified as borderline (7 studies) and average (5 studies). Samples comprised a total of 1,361 participants assessed for the PIQ, and those with the lowest (74.76 ± 9.01)27 and highest (97.67 ± 12.0)35 PIQ score are the same as those with the lowest and highest total IQ score, respectively. Figure 2 shows results from samples assessed for verbal, performance, and total quotients, indicating the distribution by percentiles of mean and standard deviation.
Collected data are provided in Figure 2 by the variation and concentration of results, defining the maximum and minimum values as well as the mean found. The means and standard deviation found in the samples assessed indicated a higher concentration of results classified as average in verbal IQ, as well as the highest number of results classified as low average in performance IQ and total IQ (Figure 2).
Analysis of Sample Performance in the WAIS Subtests
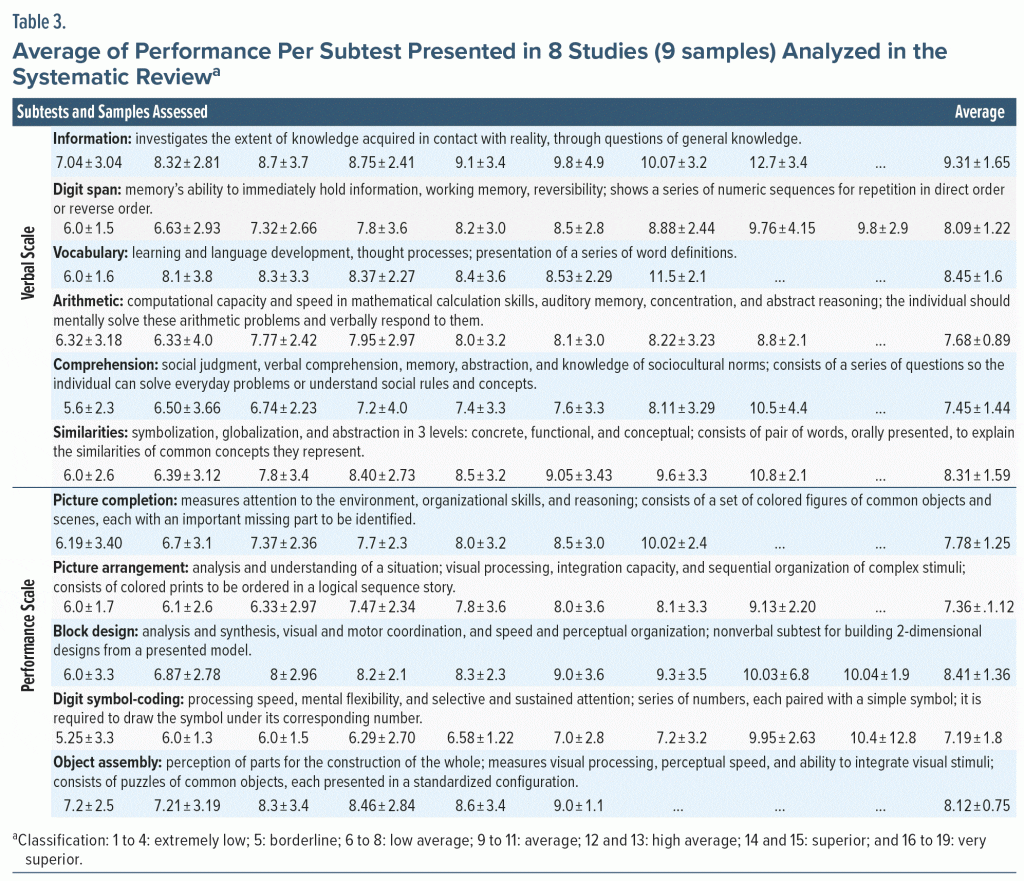
WAIS editions used in this study included 6 subtests for the verbal scale and 5 subtests for the performance scale (Table 3). Eight articles were found that described the sample’s average result in each subtest applied, resulting in 300 assessed participants, which allowed performance analysis on cognitive functions in each subtest (Table 3).
The 2 lowest scores are related to the subtests included in the performance scale (picture arrangement and digit symbol coding), which assess attention ability and mental control. The comprehension subtest had the third lowest performance, being considered a measure of social cognition, since it assesses the ability to analyze social context information, predict consequences, and make decisions.
Complementary Indices of the WAIS Results Analysis
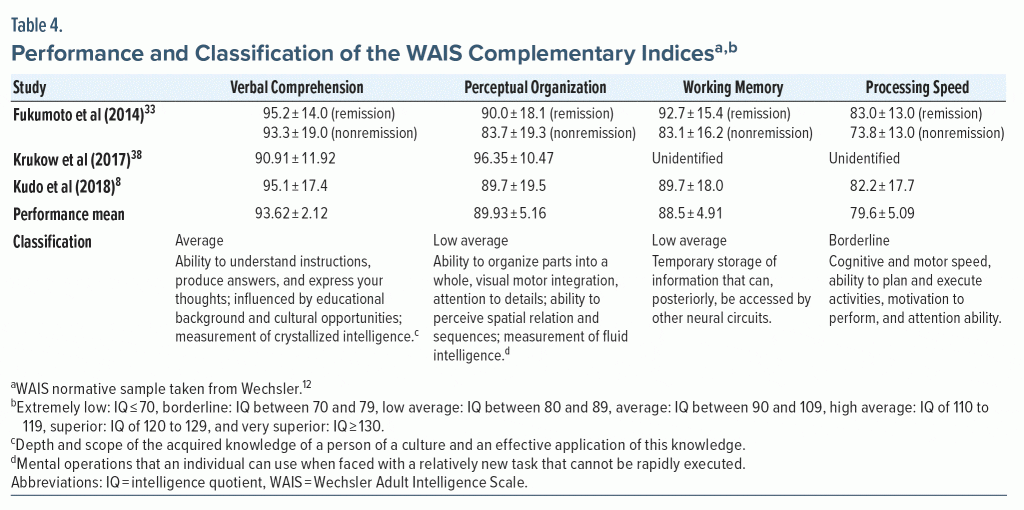
Regarding the complementary index results included in the WAIS-III, 3 studies reported results for verbal comprehension and perceptual organization index, while only 2 reported scores corresponding to working memory and processing speed indices (Table 4).
The verbal comprehension index (VCI) was classified as average, from the mean established between performances shown in the 4 samples assessed, summing a total of 360 participants (see Table 1). The lowest performance found was 90.91 ± 11.92, corresponding to a sample of 47 people.38 The highest verbal comprehension ability result was 95.2 ± 14.0 in a sample of 33 people.33
The perceptual organization index was classified as low average from the mean established for the 4 samples assessed. The lowest result classified in this index was presented by a sample of 30 people (83.7 ± 19.3).33 The highest performance classified was 96.35 ± 10.47 found in a sample of 47 people.38
Working memory index (WMI) and processing speed index (PSI) values were presented in 2 studies, with a total of 3 samples (313 participants). For these 3 studies, the mean was classified as low average for WMI and as borderline for PSI. The lowest WMI and PSI (WMI: 83.1 ± 16.2 and PSI: 73.8 ± 13.0) values were found in a sample of 30 people.33 Meanwhile, another sample of 33 patients from the same study scored higher in both indices (WMI: 92.7 ± 15.4 and PSI: 83.0 ± 13.0) and showed remission of symptoms during psychiatric treatment.33
DISCUSSION
Global Cognitive Functioning in Schizophrenia
This study aimed to identify cognitive functioning characteristics in adults with schizophrenia for WAIS tasks and which cognitive functions are impaired in this population in comparison with the scale’s normative sample. Among the 28 studies included in this systematic review, most showed a total IQ performance classified as low average, an average VIQ, and a low average PIQ for individuals with schizophrenia on the WAIS. However, schizophrenia patients showed preserved performance in verbal functions (VIQ), since the mean result of the samples showed a performance compatible with those of the healthy population used in the instrument’s validation and standardization process.
Considering the concept of intelligence as a multifactorial trait, which is not always adaptive, and not showing the exclusive need for the effectiveness of abstract reasoning, intelligence corresponds to a global competence that enables the understanding of the world and the reaction to environmental requests. Thus, the IQ considers the level of that intelligence, comparing the performance of a subject of a certain age with results obtained in the assessment of a group with individuals of the same age through verbal and nonverbal tasks.12
Comparison Between Verbal and Performance Aspects
Considering the results of the analyzed studies, verbal ability (as assessed by the verbal scale subtests) showed higher scores compared to the average obtained for the performance scale. The lowest scores found in the studies were for performance scale subtests measuring attention ability and processing speed, executive functions concerning visual processing, integration capacity and sequential organization of complex stimuli, and social cognition and social judgment. Impaired social judgment and ability to understand, which are characteristic of schizophrenia, can also be related to short-term memory capacity, given the need to manipulate received information and good contact with reality for the resolution of problems.15
In tasks that investigate attentional ability and speed to process information, there is a need for mental control to select main data and establish strategies for problem resolution and speed followed by precision to stay involved throughout the execution of the activity. In schizophrenia, there can be genetically influenced changes in neural circuits of the prefrontal cortex regions, which are related to planning, attention, and memory.6 These neurofunctional impairments are reflected in impaired ability for mental manipulation and simultaneous information processing. These impairments may in turn affect maladaptive functional and social behavior, as preserved ability for social judgment and decision making are needed to manage social context demands.6
Discrepancy between scores obtained in the verbal and working memory scale subtests, which assess working memory, perceptual organization, and processing speed (part of the performance scale), were significant given that these nonverbal abilities were considered below average when compared to the instrument’s normative data. Cognitive decline in patients with schizophrenia is related to differences in other cognitive domains assessed, since processing holds a central place in the cognitive structure of this disorder. Cognitive impairment contributes to development of other neuropsychological dysfunctions, such as planning and decision-making impairment.17
Performance on verbal tests reflects semantic memory ability, appropriate verbal learning, and interest in the environment, primarily based on educational and cultural opportunities, while having less effect on attention and information manipulation skills. Cognitive impairment in schizophrenia was consistent across the studies in this review, while still independent of psychopathologic experiences. However, an association between cognition and psychotic symptoms is noted, especially in negative and disorganized dimensions, given that the duration of illness and nonremission of symptoms reflects greater impairment of cognitive functions.18
Cognitive Functioning in Schizophrenia as an Endophenotype
Studies included in this review provide an important starting point to reinforce the need to assess neuropsychological functioning. Changes in cognitive functions may occur among individuals with schizophrenia and can be found in unaffected family members. This suggests that evidence of cognitive impairment can be considered an endophenotypic characteristic in schizophrenia.40 A study by Islam et al41 reported that positive and negative symptoms of schizophrenia in individuals with moderate and severe cognitive deficits were significantly greater than in groups with mild or no cognitive impairments, emphasizing that psychotic symptoms are associated with cognitive deficits. In addition, that study41 also found a high family correlation (27%) for cognitive aspects in schizophrenia.
Schizophrenia is a complex neurodevelopmental disorder, in which cognitive deficits are some of the main characteristics and considered as potential endophenotypes.42,43 In addition to the symptoms described as diagnostic criteria, based on results from the studies included in this review, a hypothesis is raised that cognitive performance is also a significant indicator of schizophrenia, as having a neuropsychological profile with an average VIQ score and low average PIQ was persistent in the assessed samples. Deficits involving performance skills have been noted in studies such as that by Hu et al43 as an endophenotype candidate for early diagnosis of schizophrenia.
CONCLUSION
This systematic review showed specific cognitive functioning in individuals diagnosed with schizophrenia, highlighting lower than average performance compared to healthy controls regarding executive cognitive functions such as information processing, attention, and working memory capacity. These cognitive functions cover prefrontal cortex function, which is related to problems in planning and impulse control, which are characteristics present in the diagnosis of schizophrenia.
Identification of neuropsychological functioning characteristics described in this study contributes to the understanding of cognitive dynamics found in schizophrenia. This area of research may enhance diagnosis and strengthen the hypothesis that cognitive performance may be a psychopathologic expression of psychosis. In addition, based on neuropsychological function mapping, treatment and rehabilitation programs can be developed to stimulate functions that show a higher level of dysfunction, which promotes an opportunity to create resources and reduce risks of functional and social impairment. Cognitive rehabilitation tends to contribute to relapse prevention in social and executive skills necessary for labor market integration, as well as tools that enable greater functionality and consequently better quality of life. In addition, cognitive function studies of healthy adults with prefrontal cortex genetic changes may help inform future understanding of the relationship between psychosis and cognitive changes.
Limitations of this study include the presence of few articles describing subtests, as well as complementary indices, as such information would more accurately indicate aspects of the neuropsychological profile within the diagnosis of schizophrenia, offering more robust data on the existing deficits and preserved cognitive functions. Other limiting aspects of this study correspond to the extreme values of IQ performance, since the samples assessed had different profiles and varying aspects such as age, education, and duration of illness.
Article Information
Published Online: October 12, 2023. https://doi.org/10.4088/PCC.22r03456
© 2023 Physicians Postgraduate Press, Inc.
Submitted: November 28, 2022; accepted March 10, 2023.
To Cite: Bombassaro T, Carrilho CG, Peixoto C, et al. Cognition in schizophrenia: a systematic review of Wechsler Adult Intelligence Scale studies. Prim Care Companion CNS Disord. 2023;25(5):22r03456.
Author Affiliations: Dom Bosco Catholic University, Campo Grande, Brazil (Bombassaro, Carrilho); State University of Mato Grosso do Sul, UEMS, Brazil (Peixoto); Federal University of Maranhão, UFMA, Brazil (Alves); Weill Cornell Medical College, New York, New York (Kahn); Institute of Psychiatry of the Federal University of Rio de Janeiro, Brazil (Nardi, Barciela Veras).
Corresponding Author: André Barciela Veras, MD, PhD, Institute of Psychiatry of the Federal University of Rio de Janeiro (IPUB/UFRJ), Rua Raimundo Correia, 27, Copacabana, Rio de Janeiro, Brazil ([email protected]).
Relevant Financial Relationships: Dr Barciela Veras has received grant support from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro. Drs Peixoto, Alves, Kahn, and Nardi and Mss Bombassaro and Carrilho report no relevant financial relationships.
Funding/Support: None.
ORCID: Tatiane Bombassaro: https://orcid.org/0000-0002-7128-5096; Carolina Gomes Carrilho: https://orcid.org/0000-0001-9833-7645; Clayton Peixoto: https://orcid.org/0000-0002-5334-3467; Gilberto Sousa Alves: https://orcid.org/0000-0002-0463-6183; Jeffrey Paul Kahn: https://orcid.org/0000-0002-3876-3965; Antonio Egidio Nardi: https://orcid.org/0000-0002-2152-4669; André Barciela Veras: https://orcid.org/0000-0002-4986-7639
Clinical Points
- Although cognition is impaired in schizophrenia, it has a specific pattern.
- Understanding the pattern of impairment is helpful for the diagnosis of schizophrenia.
- The differentiation of cognitive impairments is useful for differential diagnosis and comorbidity assessments in clinical practice.
References (43)

- American Psychiatry Association. Diagnostic and Statistical Manual of Mental Disorders - DSM-5. Fifth Edition. Washington: American Psychiatric Association; 2013.
- Sideli L, Mule A, La Barbera D, et al. Do child abuse and maltreatment increase risk of schizophrenia? Psychiatry Investig. 2012;9(2):87–99. PubMed CrossRef
- Ruby E, Polito S, McMahon K, et al. Pathways associating childhood trauma to the neurobiology of schizophrenia. Front Psychol Behav Sci. 2014;3(1):1–17. PubMed
- Volk DW, Lewis DA. Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease. 5th ed. Academic Press; 2014.
- Nuechterlein KH, Barch DM, Gold JM, et al. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72(1):29–39. PubMed CrossRef
- Callicott JH, Mattay VS, Bertolino A, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9(1):20–26. PubMed CrossRef
- Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60(3):229–242. PubMed CrossRef
- Kudo N, Yamamori H, Ishima T, et al. Plasma levels of soluble tumor necrosis factor receptor 2 (sTNFR2) are associated with hippocampal volume and cognitive performance in patients with schizophrenia. Int J Neuropsychopharmacol. 2018;21(7):631–639. PubMed CrossRef
- Lin YT, Liu CM, Chiu MJ, et al. Differentiation of schizophrenia patients from healthy subjects by mismatch negativity and neuropsychological tests. PLoS One. 2012;7(4):e34454. PubMed CrossRef
- Lezak MD, Howieson DB, Loring DW, et al. Neuropsychological Assessment. 5th ed. Oxford University Press; 2014.
- Michel NM, Goldberg JO, Heinrichs RW, et al. WAIS-IV profile of cognition in schizophrenia. Assessment. 2013;20(4):462–473. PubMed CrossRef
- Wechsler D. Wechsler Adult Intelligence Scale: Technical and Interpretive Manual. 4th ed. San Antonio, TX: Psychological Corporation; 2008.
- Gold JM, Hurt SW. The effects of haloperidol on thought disorder and IQ in schizophrenia. J Pers Assess. 1990;54(1-2):390–400. PubMed
- Goldman RS, Axelrod BN, Tandon R, et al. Spurious WAIS-R cholinergic profiles in schizophrenia. Clin Neuropsychol. 1993;7(2):171–178. PubMed CrossRef
- Condray R, van Kammen DP, Steinhauer SR, et al. Language comprehension in schizophrenia: trait or state indicator? Biol Psychiatry. 1995;38(5):287–296. PubMed CrossRef
- Fujii DE, Ahmed I, Jokumsen M, et al. The effects of clozapine on cognitive functioning in treatment-resistant schizophrenic patients. J Neuropsychiatry Clin Neurosci. 1997;9(2):240–245. PubMed CrossRef
- Hawkins KA, Sullivan TE, Choi EJ. Memory deficits in schizophrenia: inadequate assimilation or true amnesia? findings from the Wechsler Memory Scale–revised. J Psychiatry Neurosci. 1997;22(3):169–179. PubMed
- Mortimer AM, Bowen K. Measuring IQ in schizophrenia research: an update of the Quick Test in estimating IQ decline. Cogn Neuropsychiatry. 1999;4(2):81–88. PubMed CrossRef
- Ilonen T, Taiminen T, Lauerma H, et al. Impaired Wisconsin card sorting test performance in first-episode schizophrenia: resource or motivation deficit? Compr Psychiatry. 2000;41(5):385–391. PubMed CrossRef
- Egan MF, Goldberg TE, Gscheidle T, et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50(2):98–107. PubMed CrossRef
- Baron K, Hays JR. Characteristics of readmitted psychiatric inpatients. Psychol Rep. 2003;93(1):235–238. PubMed CrossRef
- Zakzanis KK, Andrikopoulos J, Young DA, et al. Neuropsychological differentiation of late-onset schizophrenia and dementia of the Alzheimer’s type. Appl Neuropsychol. 2003;10(2):105–114. PubMed CrossRef
- Nakano H, Terao T, Iwata N, et al. Symptomatological and cognitive predictors of insight in chronic schizophrenia. Psychiatry Res. 2004;127(1-2):65–72. PubMed CrossRef
- Hori H, Noguchi H, Hashimoto R, et al. Antipsychotic medication and cognitive function in schizophrenia. Schizophr Res. 2006;86(1-3):138–146. PubMed CrossRef
- Maeda K, Kasai K, Watanabe A, et al. Effect of subjective reasoning and neurocognition on medication adherence for persons with schizophrenia. Psychiatr Serv. 2006;57(8):1203–1205. PubMed CrossRef
- Mo S, Su Y, Chan RC, et al. Comprehension of metaphor and irony in schizophrenia during remission: the role of theory of mind and IQ. Psychiatry Res. 2008;157(1-3):21–29. PubMed CrossRef
- Stefanopoulou E, Lafuente AR, Saez Fonseca JA, et al. Insight, global functioning and psychopathology amongst in-patient clients with schizophrenia. Psychiatr Q. 2009;80(3):155–165. PubMed CrossRef
- Ozguven HD, Oner O, Baskak B, et al. Theory of mind in schizophrenia and Asperger’s syndrome: relationship with negative symptoms. Klinik Psikofarmakol Bulteni. 2010;20(1):5–13. PubMed CrossRef
- Lu W, Zhang C, Yi Z, et al. Association between BDNF Val66Met polymorphism and cognitive performance in antipsychotic-naïve patients with schizophrenia. J Mol Neurosci. 2012;47(3):505–510. PubMed CrossRef
- Rapp EK, White-Ajmani ML, Antonius D, et al. Schizophrenia comorbid with panic disorder: evidence for distinct cognitive profiles. Psychiatry Res. 2012;197(3):206–211. PubMed CrossRef
- Zhu X, Gu H, Liu Z, et al. Associations between TCF4 gene polymorphism and cognitive functions in schizophrenia patients and healthy controls. Neuropsychopharmacology. 2013;38(4):683–689. PubMed CrossRef
- Kao YC, Liu YP, Lien YJ, et al. The influence of sex on cognitive insight and neurocognitive functioning in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:193–200. PubMed CrossRef
- Fukumoto M, Hashimoto R, Ohi K, et al. Relation between remission status and attention in patients with schizophrenia. Psychiatry Clin Neurosci. 2014;68(3):234–241. PubMed CrossRef
- Dang J, Zhang J, Guo Z, et al. A pilot study of iPad-assisted cognitive training for schizophrenia. Arch Psychiatr Nurs. 2014;28(3):197–199. PubMed CrossRef
- El-Missiry A, Elbatrawy A, El Missiry M, et al. Comparing cognitive functions in medication adherent and non-adherent patients with schizophrenia. J Psychiatr Res. 2015;70:106–112. PubMed CrossRef
- Nilsson BM, Holm G, Ekselius L. Karolinska scales of personality, cognition and psychotic symptoms in patients with schizophrenia and healthy controls. Nord J Psychiatry. 2016;70(1):53–61. PubMed CrossRef
- Liang S, Deng W, Wang Q, et al. Performance of verbal fluency as an endophenotype in patients with familial versus sporadic schizophrenia and their parents. Sci Rep. 2016;6(1):32597. PubMed CrossRef
- Krukow P, Karakuła-Juchnowicz H, Juchnowicz D, et al. Processing speed is associated with differences in IQ and cognitive profiles between patients with schizophrenia and their healthy siblings. Nord J Psychiatry. 2017;71(1):33–41. PubMed CrossRef
- Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed CrossRef
- Wang Q, Chan R, Sun J, et al. Reaction time of the continuous performance test is an endophenotypic marker for schizophrenia: a study of first-episode neuroleptic-naive schizophrenia, their non-psychotic first-degree relatives and healthy population controls. Schizophr Res. 2007;89(1-3):293–298. PubMed CrossRef
- Islam MA, Habtewold TD, van Es FD, et al; GROUP Investigators. Long-term cognitive trajectories and heterogeneity in patients with schizophrenia and their unaffected siblings. Acta Psychiatr Scand. 2018;138(6):591–604. PubMed CrossRef
- Heinrichs RW, Ammari N, Miles AA, et al. Cognitive performance and functional competence as predictors of community independence in schizophrenia. Schizophr Bull. 2010;36(2):381–387. PubMed CrossRef
- Hu M, Chen J, Li L, et al. Semantic fluency and executive functions as candidate endophenotypes for the early diagnosis of schizophrenia in Han Chinese. Neurosci Lett. 2011;502(3):173–177. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top