Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Graves’ disease is an autoimmune condition that presents with hyperthyroidism, goiter, ophthalmopathy, and a number of neuropsychiatric and physical signs and symptoms. Graves’ disease is the most common cause of hyperthyroidism. Women are affected 5 to 10 times more often than men.
Delusional Psychosis in Graves’ Disease
To the Editor: Graves’ disease is an autoimmune condition that presents with hyperthyroidism, goiter, ophthalmopathy, and a number of neuropsychiatric and physical signs and symptoms. Graves’ disease is the most common cause of hyperthyroidism.1,2 Women are affected 5 to 10 times more often than men.1,3 The prevalence of Graves’ disease is about 60%-80% in hyperthyroid patients, affirming its preponderance in hyperthyroid conditions.1 While the psychiatric symptoms in these patients vary immensely, the presence of delusions as the only psychiatric presentation of Graves’ disease appears to be extremely rare. In this case report, we describe such an atypical psychosis—a psychotic disorder with prominent delusions—in a patient with hyperthyroidism secondary to Graves’ disease.
Case report. Ms A is a 58-year-old black woman who was brought to acute psychiatric services in the emergency department of an urban tertiary care hospital by the police after she was found crying on the street with the intent to jump off a bridge. She alleged that she was raped in her apartment by a young neighbor who lived upstairs. She claimed on various occasions that he entered her house "through the window using a ladder," "through a piece of plywood in the closet," and "through a hole in the wall." She also remarked, "I wake up smelling like pure sex," and "The blood thinner meds I take for my stroke put me into a deep sleep; I cannot hear or feel anything because of the medication" (the patient was actually taking aspirin and not blood thinners). Ms A reported taking 325 mg of aspirin and atorvastatin 20 mg daily and denied the use of illicit substances. A review of systems was negative for recent symptoms of mania, obsessive-compulsive disorder, or neurovegetative depression but was positive for weight loss, palpitations, and occasional night sweats. She had not slept consistently well in several days because she was afraid of her neighbor. Ms A kept a knife under her pillow to protect herself from the "rapist" and had a plan to either stab him or set fire to his truck. She had changed the locks and made several police reports, but she was tearful and frustrated that "the police found no evidence of forced entry, so they think I am making this up."
At the time of admission, Ms A was found to have a blood alcohol concentration of 0.063%, but her drug screening immunoassay and gas chromatography/mass spectrometry results were negative. She was interviewed after the active influence of alcohol had cleared and continued to make the same statements with accompanying distress. Her mental status examination was significant for poor grooming and lack of self-care, excessive tearfulness, dysphoric mood, paranoia about the alleged rape, poor insight and judgment about her situation, and difficulty coping. She reported no abnormal sensory perceptions and exhibited no response to internal stimuli. Ms A had preserved thought form, and her communications were largely goal-directed with only mild circumstantiality. Her thought content was concerning for ongoing passive suicidal ideas and active violent urges toward her neighbor. Ms A was admitted to the inpatient psychiatry department. Her severe affective and psychotic symptoms and impaired insight led clinicians to conclude that someone might come to imminent harm because she might act on her suicidal ideation or violent thoughts about her neighbor, the alleged rapist. At that time, she was diagnosed with delusional disorder, persecutory type (DSM-5 criteria).
A review of her medical records revealed another visit to acute psychiatric services 17 months prior when Ms A reported that she was being raped by her neighbor; however, police found no evidence of forced entry into her apartment. During the previous visit, she was examined by a nurse specializing in sexual assault services who found no physical evidence of rape. Previous psychiatric diagnoses for Ms A included major depressive disorder and cocaine use disorder, both in full remission for over 10 years prior to her current admission. Her medical history was significant for a right hemispheric stroke in the parietotemporal region secondary to cocaine use, which was noted to be consistently stable over time with no changes as shown on repeated imaging performed for unrelated complaints.
As part of the workup for Ms A’s review of systems, thyroid function testing was added to standard baseline laboratories. Results of the blood chemistries and hemogram were unremarkable, but endocrinologic investigations were significant for a low thyroid-stimulating hormone level of < 0.1 mIU/L (normal: 0.3 to 4.2 mIU/L), elevated free T3 level of 6.6 pg/mL (normal: 2.0 to 4.4 pg/mL), and an elevated free T4 level of 2.6 ng/dL (normal: 0.8 to 1.6 ng/dL). Subsequent testing showed the thyroid peroxidase antibody level was markedly elevated at > 1,087 IU/mL (normal: 0 to 3.9 IU/mL), and the thyroid-stimulating immunoglobulin level was elevated at 139% (normal: ≤ 122%). A computed tomography (CT) scan of the head revealed a stable chronic right middle cerebral artery distribution infarct with unchanged encephalomalacia involving the right frontal, parietal, and temporal convexities. There were also tiny bilateral, peripheral cerebellar hemisphere infarcts of unclear age and significance (not shown).
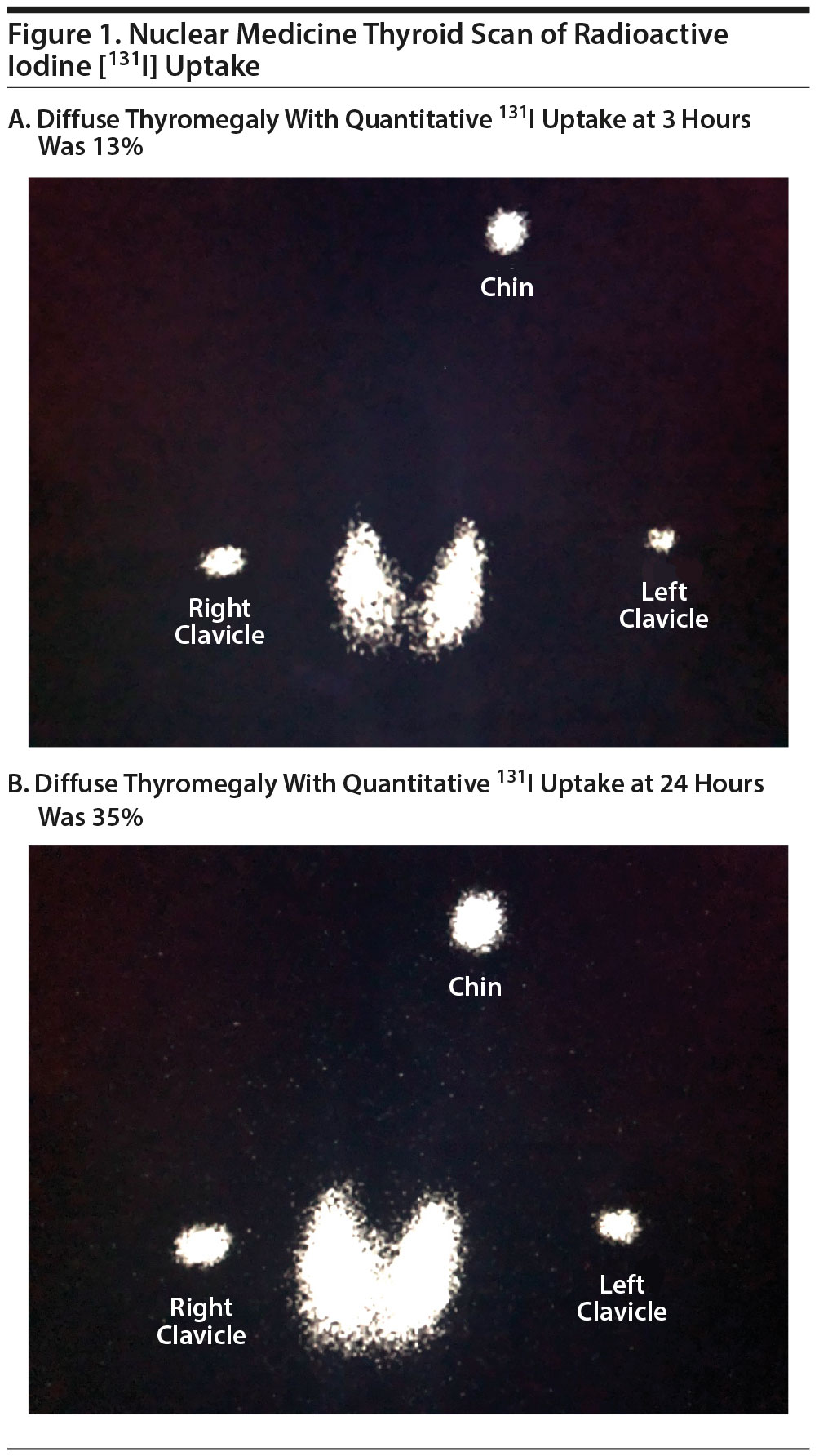
The consulting endocrinology service recommended pursuing a diffusion uptake scan of the thyroid gland, which showed mildly elevated uptake of iodine: 13% at 3 hours and 35% at 24 hours (Figure 1). There was also moderate, diffuse thyromegaly, with the overall gland size enlarged by a factor of 2 to 2½ times the normal size. A diagnosis of Graves’ disease (ICD-9 code E05) was made. Ms A was prescribed propranolol 40 mg twice daily and methimazole 5 mg daily, which she continued to take at the time of hospital discharge. The primary treatment team recommended symptomatic intervention with antipsychotic medications, but she was resistant to taking any psychotropics. Throughout the hospital stay, Ms A continued to appear delusional about having suffered sexual assaults, but she became less fixated on the assaults and was less distressed by them. At the time of discharge after 6 days of inpatient hospitalization, there were no suicidal or violent thoughts, and she was discharged home with follow-up scheduled with the endocrinologist.
Three months later at her first follow-up after inpatient psychiatric hospitalization, Ms A was seen in the outpatient setting by an endocrinologist who documented no delusional thought processes. Her TSH level at that time was still < 0.1 mIU/L, but the free T4 level had normalized to 1.5 ng/dL. She was taking propranolol and methimazole inconsistently, but she was largely asymptomatic. In light of her poor medication adherence, a radioiodine ablation was recommended and successfully completed. Ms A was admitted to the hospital 4 months later for aphasia, right facial droop, and limb weakness, all of which resolved over the course of a brief hospital stay. At that time, her CT scan remained unchanged (not shown). Her TSH level was 3.4 mIU/L, and she remained otherwise free of symptoms of hyperthyroidism. Ms A was seen by the consultation-liaison psychiatry service during that admission, and no delusions or other psychiatric symptoms were noted. On the basis of the course of illness and available data, Ms A’s delusional disorder was deemed secondary to hyperthyroidism, which was, in turn, caused by Graves’ disease. She has had no further documented contact with our medical facilities.
This case report illustrates an unusual presentation of a pure delusional disorder due to hyperthyroidism. Hyperthyroid patients may be expected to present with a variety of physical symptoms (Table 1). Along with cardiac, structural, and constitutional problems, the clinical picture may include diverse ophthalmologic manifestations. Moreover, patients may report neuropsychiatric issues with deficiencies in cognitive functioning such as decreases in attention, concentration, memory, planning, and overall productivity.4,5 While mood and anxiety syndromes, such as major depressive disorder, generalized anxiety disorder, panic disorder, social anxiety disorder, and even bipolar disorder have been well documented in the literature on hyperthyroidism, psychotic symptoms are a rare phenomena in hyperthyroid states.1,4,6
Lo et al7 found that organic delusional disorders are very rare and constitute 2.9% of mental illnesses due to underlying organic causes, leading to just 0.4% of total psychiatric admissions. Furthermore, hyperthyroidism is a very rare cause of organic delusional disorders; of the 22 patients with organic delusional disorders studied by Lo et al,7 4 had psychosis caused by hyperthyroidism. Brownlie et al8 identified 18 patients over a 20-year period with psychosis associated with hyperthyroidism. Of these patients, 13 had delusions, but they occurred in conjunction with other psychiatric symptoms such as depression, mania, and hallucinations. The majority had a significant affective component to their mental symptomatology, and 2 had no psychosis at initial presentation but developed "severe mood swings" with associated psychosis after commencement of treatment when thyroid hormone levels started falling.8 Thus, isolated delusional psychosis is a rare phenomenon in the context of hyperthyroidism. It is unclear whether the cause of a thyrotoxic state (eg, Graves’ disease vs Hashimoto’s thyroiditis vs toxic goiter vs exogenous thyroid hormone poisoning) contributes to the likelihood of developing psychosis or affects the particular constellation of psychiatric symptoms.8,9
Hyperthyroidism is established and further investigated through a combination of blood tests and imaging studies. The hyperthyroidism in Graves’ disease may be overt with low serum TSH and high thyroid hormone concentrations or more subclinical with low serum TSH alone.1,2,8 Even patients with no laboratory-confirmed thyroid hormone excess may exhibit neuropsychiatric and physical abnormalities, although symptoms are likely to be less intense.6 Thyroid scans may show abnormalities such as diffuse hyperplasia and toxic goiters—solitary or multinodular.8 Immunologic studies like assays of antibodies to thyroglobulin and thyroid peroxidase may be positive, but these results are not firmly diagnostic.2
Ms A’s physical symptoms of sleep disturbance, weight loss, palpitations, and night sweats prompted investigation into an underlying thyroid disorder. The uniqueness of her presentation stems from the prominent delusional symptoms. Although she did have a dysphoric mood, paranoia, and tearfulness, these symptoms were considered to be consequences of her delusional thought content rather than representative of a different, more common psychiatric condition. The DSM-510 requires the presence of delusion(s) for a period of 1 month or longer to make the diagnosis of delusional disorder with a relative preservation of previous level of functioning and exclusion of the diagnosis of schizophrenia. Ms A’s dramatic delusions persisting for at least 17 months in the absence of other symptoms of schizophrenia initially led to a diagnosis of unspecified delusional disorder. Her persecutory delusions were the most prominent symptom, and the time frame was consistent with diagnostic criteria. Even though delusional disorders may be fairly resistant to treatment with neuroleptics,11 medications were offered due to the patient’s acute distress. Ultimately, the diagnosis was revised to psychotic disorder with delusions due to hyperthyroidism secondary to Graves’ disease, because the investigatory evidence pointed to an underlying hyperthyroid state (elevated TSH and thyroid hormone levels), and the treatment of said state led to significant abatement of symptoms. Ms A had no neurologic signs or symptoms suggestive of a stroke, and head imaging revealed no new cerebrovascular abnormalities that would explain her clinical features. We acknowledge that Ms A’s previous cerebrovascular accident may have rendered her more vulnerable to the development of such a singular symptom complex informed, phenomenologically, by her psychosocial situation.
There are no established guidelines for the management of psychosis in the context of hyperthyroidism. Our literature review revealed a few case reports12-14 describing the use of typical antipsychotics such as haloperidol and atypical antipsychotics such as olanzapine and aripiprazole. Regardless of the utilization of antipsychotics, the definitive treatment of symptomatic manifestations of hyperthyroidism in Graves’ disease involves addressing the underlying thyroid dysfunction through antithyroid medications such as propylthiouracil and methimazole, ablation with radioactive iodine-131, or thyroid surgery.2,6 For accompanying affective and anxiety problems, lithium (noting its propensity to inhibit thyroid function15) may be considered for its mood-stabilizing properties, and propranolol may be helpful to manage anxiety while diminishing peripheral effects of thyroid excess,1,16 but definitively treating the underlying hyperthyroid state should be viewed as the core psychiatric treatment. In the case of Ms A, a fairly rapid improvement in symptoms was achieved with medical management using propranolol and methimazole.1,8 At no point did she receive any antipsychotic medications. Ultimately, she underwent radioiodine ablation that resulted in complete resolution of her delusional symptoms.
This case report describes an unusual delusional psychosis in the setting of hyperthyroidism secondary to Graves’ disease. The neuropsychiatric manifestations of hyperthyroidism are manifold. In the presence of other neuropsychiatric and physical signs and symptoms, it is important to investigate for the presence of an underlying hyperthyroid state and manage it accordingly. In light of the absence of recommended guidelines, the decision to treat with psychiatric medications needs to be tailored to patients’ individual clinical presentations. With no definitive somatic intervention, the efficacy of symptom management with psychotropic agents is unclear.
References
1. Bunevicius R, Prange AJ. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006;20(11):897-909. PubMed CrossRef
2. Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;168(5):575-585. PubMed
3. Vanderpump MPJ. The epidemiology of thyroid disease. In: Braverman LE, Utiger RD, eds. Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2005:398-406.
4. Trzepacz PT, Klein I, Roberts M, et al. Graves’ disease: an analysis of thyroid hormone levels and hyperthyroid signs and symptoms. Am J Med. 1989;87(5):558-561. PubMed CrossRef
5. Stern RA, Robinson B, Thorner AR, et al. A survey study of neuropsychiatric complaints in patients with Graves’ disease. J Neuropsychiatry Clin Neurosci. 1996;8(2):181-185. PubMed CrossRef
6. Bunevicius R, Velickiene D, Prange AJ. Mood and anxiety disorders in women with treated hyperthyroidism and ophthalmopathy caused by Graves’ disease. Gen Hosp Psychiatry. 2005;27(2):133-139. PubMed CrossRef
7. Lo Y, Tsai SJ, Chang CH, et al. Organic delusional disorder in psychiatric in-patients: comparison with delusional disorder. Acta Psychiatr Scand. 1997;95(2):161-163. PubMed CrossRef
8. Brownlie BEW, Rae AM, Walshe JWB, et al. Psychoses associated with thyrotoxicosis—"thyrotoxic psychosis": a report of 18 cases, with statistical analysis of incidence. Eur J Endocrinol. 2000;142(5):438-444. PubMed CrossRef
9. da Silva JA, Almeida JT, Corrêa BB, et al. Acute psychotic episode in a patient with thyrotoxicosis factitia [published online March 17, 2009]. BMJ Case Rep. 2009;2009:bcr0820080676. PubMed CrossRef
10. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
11. Mu×±oz-Negro JE, Cervilla JA. A systematic review on the pharmacological treatment of delusional disorder. J Clin Psychopharmacol. 2016;36(6):684-690. PubMed CrossRef
12. Macedo LR, Marino J, Bradshaw B, et al. Graves’ hyperthyroidism-induced psychosis treated with aripiprazole: a case report. 2012;26(1):59-61.
13. Emul M, Sakalli A, Erol TC, et al. Thyrotoxic psychosis in an elderly woman and haloperidol use: a case report. Psychogeriatrics. 2013;13(1):49-51. PubMed CrossRef
14. Dahale AB, Chandra PS, Sherine L, et al. Postpartum psychosis in a woman with Graves’ disease: a case report. Gen Hosp Psychiatry. 2014;36(6):761.e7-761.e8. PubMed CrossRef
15. Prakash I, Nylen ES, Sen S. Lithium as an alternative option in graves thyrotoxicosis [published online September 6, 2015]. Case Rep Endocrinol. 2015;2015:869343. PubMed CrossRef
16. Abraham P, Acharya S. Current and emerging treatment options for Graves’ hyerthyroidism. Ther Clin Risk Manag. 2010;6:29-40. PubMed
aDepartment of Psychiatry, Hennepin County Medical Center, Minneapolis, Minnesota
bLiaison Psychiatry, Hennepin County Medical Center, Minneapolis, Minnesota
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Permission was obtained from the patient to publish this case.
Published online: January 25, 2018.
To cite: Adediran KI, Alapati D, Rasimas JJ. Delusional psychosis in Graves’ disease. Prim Care Companion CNS Disord. 2018;20(1):17l02145.
To share: https://doi.org/10.4088/PCC.17l02145
© Copyright 2018 Physicians Postgraduate Press, Inc.
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top