ABSTRACT
Objective: To estimate the prevalence and predictors of metabolic syndrome among substance users in North India.
Methods: A total of 302 participants with a history of substance use (per ICD-10 code) visiting either medicine or psychiatry outpatient departments and referred to a deaddiction center in the psychiatry department of a tertiary care hospital were enrolled. The cross-sectional study was conducted over 6 months between September 2019 and February 2020. Information regarding sociodemographic profiles was collected. Weight, height, waist circumference, hip circumference, and blood pressure were measured. A fasting venous blood sample was collected to measure blood glucose; triglycerides; high-density, low-density (LDL), and very low-density lipoprotein (VLDL) levels; and other blood parameters. The International Diabetes Federation criterion was used to define metabolic syndrome. Descriptive analysis was performed, and multiple logistic regression was used.
Results: The mean ± SD age of the study participants was 37.1 ± 11.4 years, and the majority were males (n = 299, 99.0%). The prevalence of metabolic syndrome among substance users was 16.9% (n = 51). Mean age, age of initiation, weight, body mass index (BMI), hip circumference, total cholesterol, LDL, and VLDL were significantly higher (all P < .05) among study participants with metabolic syndrome than among those without. On multivariable regression analysis, professional employment, high BMI, high hip circumference, and elevated VLDL were predictors of metabolic syndrome among substance users.
Conclusions: Coexisting substance use and metabolic syndrome is a public health concern considering the large number of people who are substance users. It is essential to screen such patients regularly for cardio-vasculo-metabolic disorders to prevent further morbidity and mortality.
Prim Care Companion CNS Disord 2022;24(4):21m03172
To cite: Verma M, Govil N, Chahal S, et al. Determinants of metabolic syndrome among people with substance abuse: a cross-sectional study from North India. Prim Care Companion CNS Disord. 2022;24(4):21m03172.
To share: https://doi.org/10.4088/PCC.21m03172
© 2022 Physicians Postgraduate Press, Inc.
aDepartment of Community and Family Medicine, All India Institute of Medical Sciences, Bathinda, Punjab, India
bDepartment of General Medicine, Kalpana Chawla Government Medical College, Karnal, Haryana, India
cDepartment of Psychiatry, Kalpana Chawla Government Medical College, Karnal, Haryana, India
dDepartment of Community Medicine, North Delhi Municipal Corporation Medical College and Hindu Rao Hospital, Delhi, India
eBharti Hospital, Karnal, India
‡Authors contributed equally.
*Corresponding author: Priyanka Sharma, MD, Department of Community Medicine, North Delhi Municipal Corporation Medical College and Hindu Rao Hospital, G Block, 6th Fl, New Delhi 110007 India ([email protected]).
Psychoactive drugs are substances that, when taken in or administered into one’s system, affect mental processes, eg, perception, consciousness, cognition or mood, and emotions. According to the World Health Association,1 about 270 million people (or about 5.5% of the global population aged 15–64 years) had used psychoactive drugs in the previous year, and about 35 million people are estimated to be affected by drug use disorders (harmful pattern of drug use or drug dependence). The use of various psychoactive substances such as alcohol, cannabis, and opioids has been observed in India for centuries. As per a recent national-level survey from India,2 alcohol is the most common substance used, followed by cannabis and opioids. The prevalence of alcohol use is 4.6%, with a male:female ratio of 17:1, followed by cannabis at 2.8% and opioids at 2.1%.2
Although the burden is substantial, the current dimensions of the effects and implications of their use are not well documented. These effects are drug and dose dependent, apart from the user’s health. Short-term effects can range from just changes in appetite and mood to cardiac or cerebrovascular events, psychosis, and even death. Longer-term effects can lead to addiction, which can cause infections like HIV/AIDS, hepatitis, mental illness, and other cardiovascular-metabolic disorders.3 Alcohol and tobacco use, in particular, has been associated with increased risk of diabetes, cardiovascular mortality, and the development of metabolic syndrome.4,5 Metabolic syndrome, in turn, is a significant risk factor for cardiovascular diseases and all-cause mortality.6 Metabolic syndrome is a cluster of abnormalities that include insulin resistance, hypertension, dyslipidemia, and abdominal obesity.6 The prevalence of metabolic syndrome in the substance-dependent population has been reported in the range of 5%–31%.7
Patients with substance use have an increased risk of metabolic syndrome due to nutritional deficiencies, which increase cell damage, augment excitotoxicity, reduce energy production, and lower the antioxidant potential of the cells.8 Although their relationship remains complex, low to moderate alcohol use has been found to decrease the risk for metabolic syndrome, while heavy alcohol use increases risk.9 Another study10 reported an increased risk of metabolic syndrome even with low alcohol use. Alcohol also affects the body mass index (BMI), and a higher BMI contributes to metabolic syndrome. There is minimal research depicting an association between metabolic syndrome and substance abuse from North India. The theme for the 2020 United Nations International Day Against Drug Abuse and Illicit Trafficking was “Better Knowledge for Better Care,” which indicates the importance of scientific evidence for enhanced response to the global drug problem and to those in need.11 Therefore, the present study was conducted among patients with a history of substance abuse visiting a tertiary care center of North India with a primary objective of estimating the prevalence and predictors of metabolic syndrome.
METHODS
Study Design
The present study used a cross-sectional design and was conducted over 6 months between September 2019 and February 2020.
General and specific study setting. The study was conducted in a newly established premier tertiary care center of Haryana, a northern state of India. This medical college caters to the needs of approximately 4 adjoining districts of Haryana and 3 districts of Uttarakhand and Uttar Pradesh. The medical college specializes in preventive, promotive, and curative services with a professional and dedicated staff. According to the 2018 records, the medical college provided care to 5,76,651 outpatients and 36,656 inpatients, including those admitted to the emergency department of the medical college. Within the medical college, the study was conducted in the deaddiction center of the psychiatry department. The department receives patients directly or through a referral from various departments. In the department, patients receive therapeutic and counseling services regarding deaddiction.
Study Population
Patients with substance dependence visiting either the department of psychiatry or the department of medicine of the study hospital on an outpatient basis who were referred to the deaddiction center were included in the study. Participants who were on treatment with any kind of regular medication other than that used to treat any of the components of metabolic syndrome and participants not following the necessary instructions required for carrying out laboratory investigations like overnight fasting were excluded from the study.
Sample Size and Sampling Strategy
The sample size was calculated using an online sample size calculator (https://www.openepi.com/SampleSize/SSPropor.htm). A sample size of 302 was calculated using the single population proportion formula after considering the prevalence of metabolic syndrome to be about 13% as per a previous study from North India,7 with a 95% confidence interval and a margin of error of 5%. Patients with substance dependence were recruited from the outpatient setting at their first contact at either the department of psychiatry or the department of medicine. The diagnosis was made per the ICD-10 Classification of Mental and Behavioral Disorders Clinical Descriptions and Diagnostic Guidelines.12 Eligible patients were invited to participate in the study through a systematic random sampling technique. The sampling interval was determined by dividing the average number of yearly deaddiction consultations by its sample size. The first participant was selected by lottery method from the order of their daily registration number. Then, every fourth participant at the exit of the outpatient department was included in the study. If the fourth patient was ineligible, unwilling to participate, or did not have reports required for the diagnosis of metabolic syndrome, the subsequent patient was considered. The process was repeated until the requisite sample size was achieved.
Study Instrument
The data were collected using a structured data collection tool. The tool included 2 broad components. Part A collected data regarding the sociodemographic details, history, and pattern of substance abuse. At the same time, part B collected data about the metabolic and anthropometric components of metabolic syndrome. Anthropometry was done using standard protocols.13 Calibrated scales measured body weight in kilograms and height in centimeters. Waist circumference was measured in centimeters midway between the inferior costal margin and the superior border of the iliac crest at the end of normal expiration in a standing position. At least 2 readings at 5-minute intervals were recorded for blood pressure (BP) using a standard nonmercury manometer in a supine position. If BP was high (≥ 140/90 mm Hg), then a third reading was taken after 30 minutes; the lowest of these readings was recorded. A fasting venous blood sample was collected under the aseptic condition to measure fasting blood glucose, triglyceride, and high-density lipoprotein (HDL) levels.
Metabolic syndrome was diagnosed using International Diabetes Federation (IDF) criteria.14 According to IDF criteria, a person is considered to have metabolic syndrome if there is high waist circumference (≥ 80 cm for females and ≥ 90 cm for males of Asian origin) along with 2 of the following criteria: systolic BP ≥ 130 mm Hg with or without diastolic BP ≥ 85 mm Hg (or on treatment for hypertension), triglyceride levels ≥ 150 mg/dL (or on specific treatment for this abnormality), HDL cholesterol < 40 mg/dL for males and < 50 mg/dL for females (or on specific treatment for this abnormality), and fasting blood glucose ≥ 100 mg/dL (or on treatment for diabetes mellitus).
Analysis and Statistics
Quantitative data were double entered, validated, and analyzed using EpiData version 3.1 and 2.2.2.182 (EpiData Association, Odense, Denmark). Key analytic outputs were the number and proportion of patients suffering from metabolic syndrome. Frequencies with percentages were calculated for categorical variables and mean and standard deviation (SD) for continuous variables. χ2 test, t test, and 1-way analysis of variance were used for comparisons. Binary logistic regression was performed to examine the influence of independent variables on metabolic syndrome.
Ethics Approval
Ethics approval was obtained from the Institutional Ethics Committee of the Kalpana Chawla Government Medical College, Karnal (Haryana), India (KCGMC/IEC/2019/19). Written informed consent was obtained from participants before data collection and examination. The consent form had 2 parts: information for the participant and the actual consent form, which the participant signed in the presence of a witness.
RESULTS
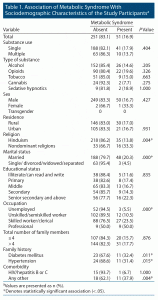
A total of 302 participants were included in the study. The mean ± SD age of the study participants was 37.1 years ± 11.4 (range, 18–80 years). Analysis of the sociodemographic data showed that most of the participants were male (n = 299, 99.0%) and followed Hinduism (n = 253, 83.8%). The most used substance was alcohol (n = 178, 58.9%), followed by opioids (n = 112, 37.1%), tobacco (n = 60, 19.9%), cannabis (n = 26, 8.6%), and sedative-hypnotics (n = 11, 3.6%) (Table 1). Of the 302 participants, 51 (16.9%) had metabolic syndrome. The following variables were significantly associated with having metabolic syndrome: nondominant religion including Sikhism and Islam, married, employed in a professional job, and family history of diabetes mellitus and hypertension (P < .05) (Table 1).
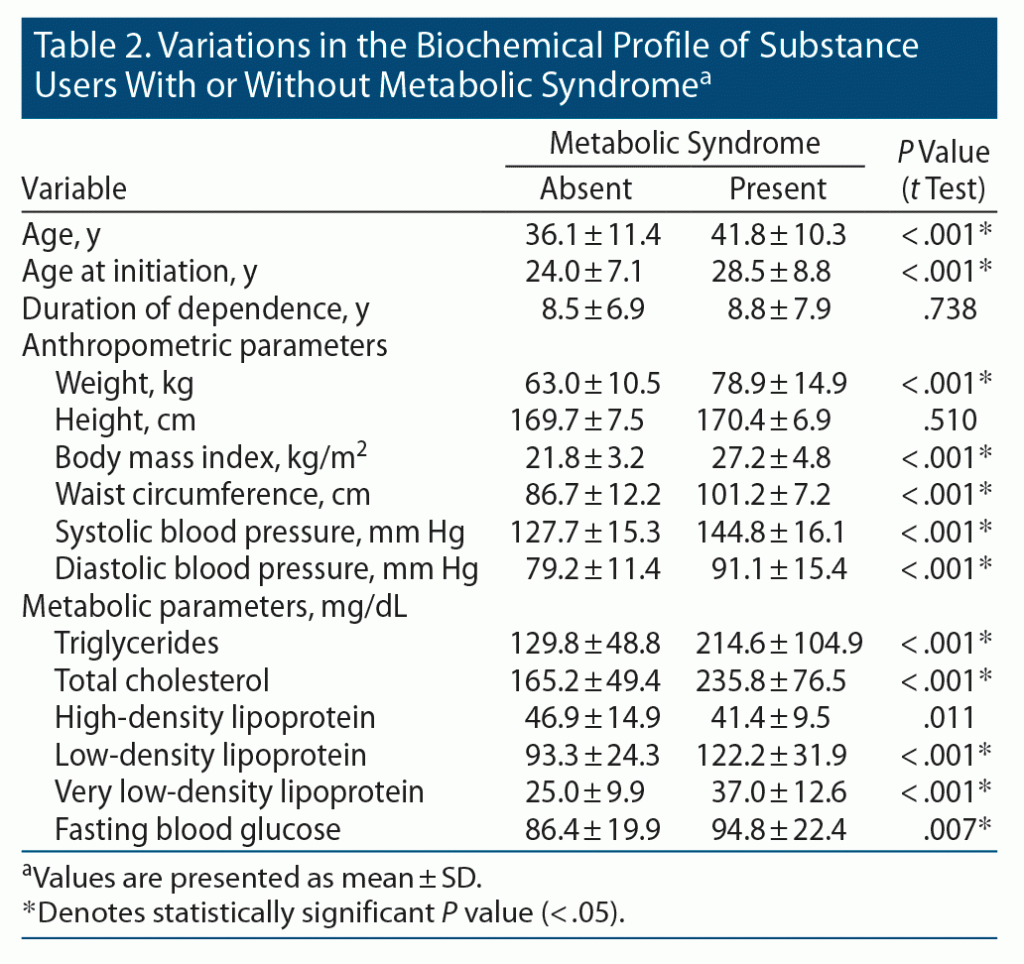
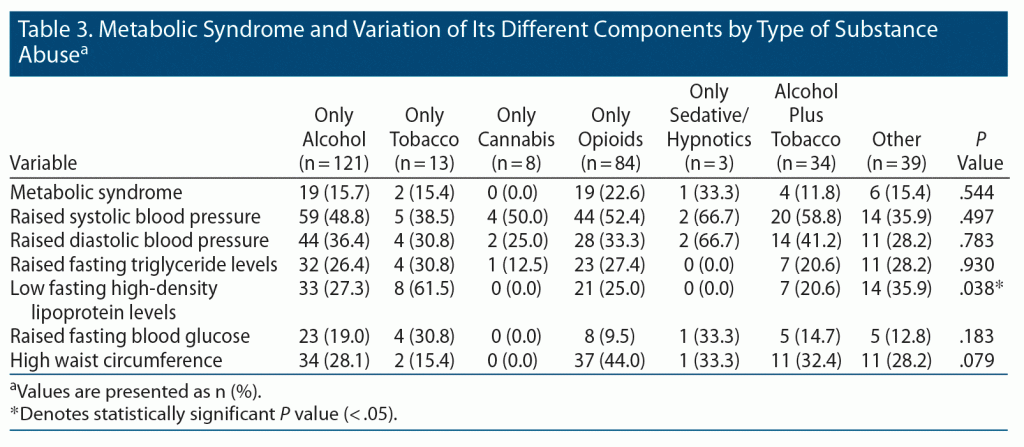
We then compared the anthropometric and biochemical parameters of participants with and without metabolic syndrome. Mean age, age of initiation, weight, BMI, hip circumference, total cholesterol, LDL, and VLDL were significantly higher among study participants with metabolic syndrome than among those without (P < .05) (Table 2). A higher percentage of tobacco users was found to have low fasting HDL levels compared to other drug users (P < .05) (Table 3).
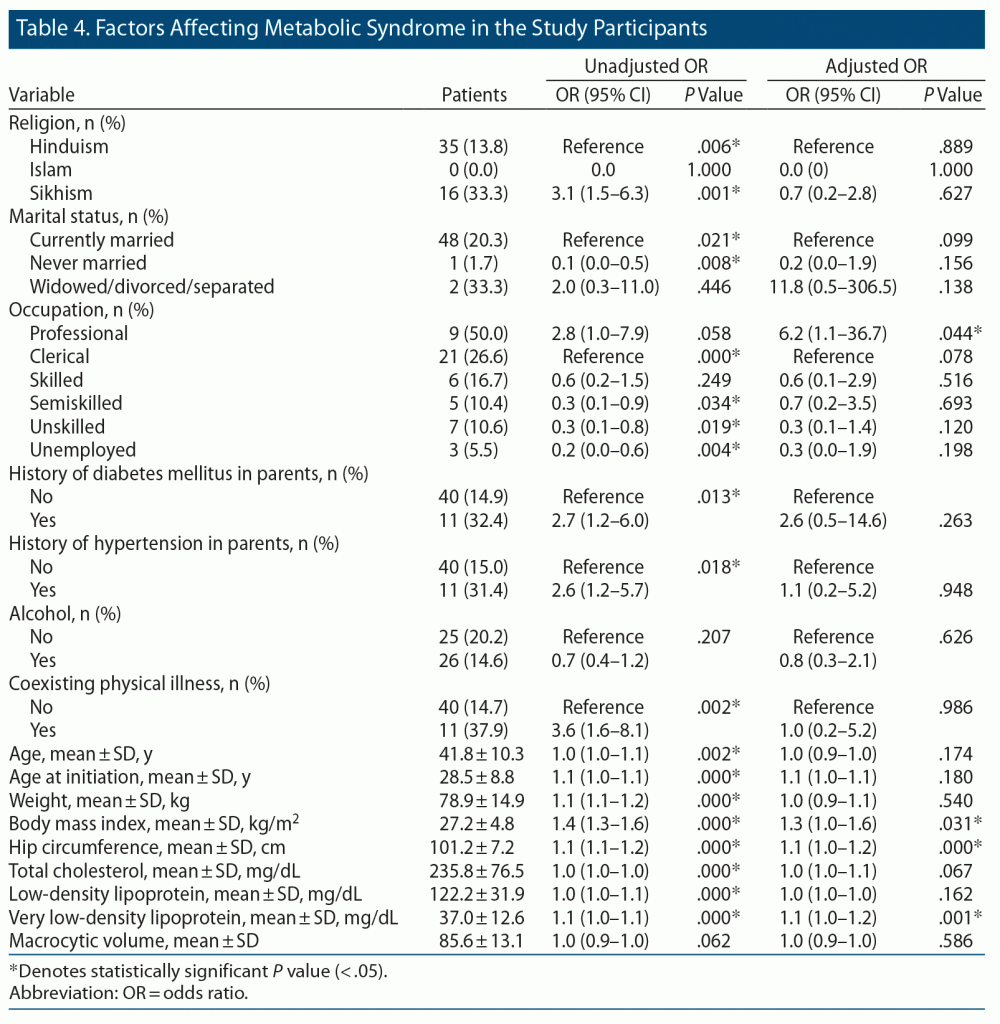
On multivariable analysis, study participants in professional jobs were found to have 6 times higher odds of metabolic syndrome than those employed in clerical jobs, and this was a significant finding (P < .05). High BMI, high hip circumference, and elevated VLDL increased the odds of developing metabolic syndrome among substance users (P < .05) (Table 4).
DISCUSSION
We diagnosed 1 of every 6 participants (16.9%) with a history of substance abuse with metabolic syndrome. This prevalence is lower than the rate of 30% among the general population (95% CI, 28%–33%).15 This finding could be due to different criteria used to define metabolic syndrome and the inclusion of substance abusers and nonusers in their study.15 Our estimates are similar to previous systematic reviews16,17 that reported the prevalence of metabolic syndrome among patients with alcohol abuse to be less than that of the general population (21.8%, 95% CI, 19.1%–24.8%). Balhara5 reported the prevalence of metabolic syndrome among individuals with opioid-dependent syndrome to be 20.3% and 5.1% according to revised National Cholesterol Education Program Adult Treatment Panel criteria and IDF criteria, respectively. There is a scarcity of data regarding the association between metabolic syndrome and different abused substances. The toxicity profile of each substance is different, and when combined with genetic vulnerability and nutritional deficiencies, which are common among substance users, the risk of metabolic syndrome increases among them.8,18
Overall, we observed that metabolic syndrome was not significantly associated with any particular type of substance abuse disorder. However, alcohol was associated with increased BP, alteration in the lipid profile, blood glucose levels, and waist circumference. Previous studies19–21 have also shown that alcohol increases obesity and causes dyslipidemia. A significant association was found by Kim et al22 between alcohol use and metabolic syndrome. Prospective studies23–24 show that light-to-moderate alcohol intake is not associated with adiposity gain, while heavy drinking is more consistently related to weight gain. Experimental evidence is also mixed and suggests that moderate alcohol intake does not lead to weight gain over short follow-up periods. However, in India, it is observed that alcohol intake is associated with increased food consumption, which may contribute to excess caloric intake, and increased frequency can explain the increase in weight even with a moderate amount of alcohol.23–26
We also observed a high prevalence of metabolic syndrome with a history of opioid use disorder. There was a high prevalence of elevated blood pressure, and around 1 in 4 participants had dyslipidemia. Fasting blood glucose was found in only one-tenth of the participants. Most clinical reports imply that opium has no remarkable impact on blood glucose, whether in nondiabetic or diabetic individuals.27–30 But, a study by Azod et al31 indicated that opium positively impacts the reduction of fasting blood glucose levels. The reduction of glucose levels has been attributed to decreased gastric emptying due to opioid μ-receptor activation, subsequently delaying the intestinal glucose absorption.31 Some clinical studies32–34 have shown that opium may affect total cholesterol, triglycerides, LDL cholesterol, and HDL cholesterol. Overall, the literature suggests that opium consumption is either ineffective or has an unfavorable impact on serum lipids. Similarly, we observed an association between tobacco use and most of the components of metabolic syndrome, which is in line with previous evidence.5,35–37
Body weight was also not associated with metabolic syndrome after adjusting for confounders. This finding was distinct from other studies7,38,39 that found higher body weight was associated with metabolic syndrome. However, there were significant differences between the BMIs of participants with and without metabolic syndrome, which corresponds to results from previous studies40 that depicted a similar pattern. Higher BMI significantly increased the odds of having metabolic syndrome in our study, and the findings are consistent with previous reports.7,38 However, individuals with average body weight as per BMI might have a high body fat percentage and could show increased metabolic dysregulation. Thus, an updated definition of obesity based on adiposity should be adopted, as it is no longer dependent on weight.41 The effect of opioids on BMI has also been evaluated in previous studies,42 but the results are inconsistent. Nevertheless, the opioid antagonist naltrexone holds promise for weight loss in obese patients. Higher VLDL was also a significant predictor of metabolic syndrome in the present study. Other biochemical factors were not associated with metabolic syndrome.
There was no significant association between age and metabolic syndrome, akin to another study39 conducted in Germany among alcohol-dependent patients and a meta-analysis conducted by Vancampfort et al.16 However, this result was in contrast with the findings of Mattoo et al7 and Bathla et al,38 who found a higher mean age among those with metabolic syndrome compared to those without. The prevalence of metabolic syndrome is higher among men aged < 50 years and women aged > 50 years. As the number of female substance users was lower in our study, the association between sex and metabolic syndrome among substance users could not be explored. Previous studies39 have found sex to be associated with metabolic syndrome. Genetic and biological factors, dietary habits, and sociocultural behaviors are associated with this age- and sex-related trend in metabolic syndrome. The risk of cardiovascular events is higher among women with metabolic syndrome than in men with the disorder. Response to treatment for metabolic syndrome varies differentially by sex.43,44 No significant association was observed between the prevalence of metabolic syndrome and marital status. This finding is in contrast to that of Mattoo et al.7 Odds of having metabolic syndrome were about 6 times higher among those employed in professional jobs than in clerical occupations. Mattoo et al7 found a higher proportion of metabolic syndrome among currently employed individuals than in those who were unemployed. This finding could partly be explained by high stress levels and a more sedentary lifestyle among professional workers, which increases their risk for metabolic syndrome.
There are some strengths in our study. A comprehensive evaluation of the patients for substance abuse was done by a psychiatrist, while the diagnosis of metabolic syndrome was by made by the physician as per predefined established criteria. Adequately calculated sample size and systematic random sampling make it easy to generalize the results. We also used diagnostic criteria for metabolic syndrome suitable for the Asian Indian ethnicity. Different criteria of diagnosis of metabolic syndrome make it difficult to compare with previous studies explicitly. However, certain limitations should also be acknowledged. It is difficult to assess the temporality of association between substance abuse and metabolic syndrome due to the cross-sectional study design. As the prevalence of substance abuse is generally low among women in India, we could include only a minimal number of female participants in the study, which prevented us from depicting the effect of substance abuse on metabolic syndrome in this population. This topic requires further studies using stratified random sampling to confirm the effect of substance abuse on metabolic syndrome.
To conclude, our study showed a high prevalence of metabolic syndrome among patients with substance abuse. This relationship between substance abuse and metabolic syndrome is a public health concern considering the high number of people with substance abuse issues has the potential to take the shape of a syndemic. Therefore, we recommend regular screening of such patients for cardio-vasculo-metabolic disorders like metabolic syndrome to prevent further morbidity and mortality. Education should be an essential component of the counseling sessions with these patients. Improvement of metabolic syndrome is expected to improve substance abuse treatment outcomes.
Submitted: October 31, 2021; accepted January 21, 2022.
Published online: August 9, 2022.
Relevant financial relationships: None.
Funding/support: None.
Clinical Points
- Synchronized treatment for patients with substance use who have metabolic syndrome can improve compliance and the prognosis and treatment outcome for both illnesses.
- Public health awareness programs should focus on the connection between substance abuse and metabolic syndrome.
- Substance abuse prevention and mitigation must be an integral part of lifestyle modification in patients with metabolic syndrome.
References (44)

- World Heath Organization. Drugs [Internet]. WHO website. Accessed July 14, 2021. https://www.who.int/health-topics/drugs-psychoactive#tab=tab_2
- Ambekar A, Agrawal A, Rao R, et al. Substance Use Magnitude in India [Internet]. On behalf of the group of investigators for the National Survey on Extent and Pattern of Substance Use in India. Ministry of Social Justice and Empowerment, Government of India. Accessed July 15, 2021. https://www.muktangan.org/pdf/Magnitude_Substance_Use_India_REPORT.pdf
- National Institute on Drug Abuse (NIDA). Health Consequences of Drug Misuse [Internet]. Drug Topics website. Accessed July 16, 2021. https://www.drugabuse.gov/drug-topics/health-consequences-drug-misuse/introduction
- Kim SK, Hong S-H, Chung J-H, et al. Association between alcohol consumption and metabolic syndrome in a community-based cohort of Korean Adults. Med Sci Monit. 2017;23:2104–2110. PubMed CrossRef
- Balhara YPS. Tobacco and metabolic syndrome. Indian J Endocrinol Metab. 2012;16(1):81–87. PubMed CrossRef
- Lind L, Sundström J, Ärnlöv J, et al. A longitudinal study over 40 years to study the metabolic syndrome as a risk factor for cardiovascular diseases. Sci Rep. 2021;11(1):2978. PubMed CrossRef
- Mattoo SK, Nebhinani N, Aggarwal M, et al. Metabolic syndrome among substance dependent men: a study from north India. Ind Psychiatry J. 2013;22(1):60–64. PubMed CrossRef
- Virmani A, Binienda Z, Ali S, et al. Links between nutrition, drug abuse, and the metabolic syndrome. Ann N Y Acad Sci. 2006;1074(1):303–314. PubMed CrossRef
- Piano MR. Alcohol’s Effects on the cardiovascular system. Alcohol Res. 2017;38(2):219–241. PubMed
- Wilsgaard T, Jacobsen BK. Lifestyle factors and incident metabolic syndrome. The Tromsø Study 1979–2001. Diabetes Res Clin Pract. 2007;78(2):217–224. PubMed CrossRef
- United Nations Office on Drugs and Crime. World Drug Report 2020 (United Nations publication, Sales No. E.20.XI.6). [Internet]. 1 Executive Summary: Impact of COVID 19; Policy Implications. UNODC website. Accessed July 16, 2021. https://wdr.unodc.org/wdr2020/field/WDR20_BOOKLET_1.pdf
- Implementation of the international statistical classification of diseases and related health problems, Tenth Revision (ICD-10). Epidemiol Bull. 1997;18(1):1–4. PubMed
- Verma M, Rajput M, Sahoo SS, et al. Correlation between the percentage of body fat and surrogate indices of obesity among adult population in rural block of Haryana. J Family Med Prim Care. 2016;5(1):154–159. PubMed CrossRef
- Alberti G, Zimmet P, Shaw J, et al. The IDF Consensus Worldwide Definition of the Metabolic Syndrome [Internet]. Vol 28. Dig Dis. 2010:186–191.
- Krishnamoorthy Y, Rajaa S, Murali S, et al. Prevalence of metabolic syndrome among adult population in India: a systematic review and meta-analysis. PLoS One. 2020;15(10):e0240971. PubMed CrossRef
- Vancampfort D, Hallgren M, Mugisha J, et al. The prevalence of metabolic syndrome in alcohol use disorders: a systematic review and meta-analysis. Alcohol Alcohol. 2016;51(5):515–521. PubMed CrossRef
- Alkerwi A, Boutsen M, Vaillant M, et al. Alcohol consumption and the prevalence of metabolic syndrome: a meta-analysis of observational studies. Atherosclerosis. 2009;204(2):624–635. PubMed CrossRef
- Goncalves A, Amiot MJ. Fat-soluble micronutrients and metabolic syndrome. Curr Opin Clin Nutr Metab Care. 2017;20(6):492–497. PubMed CrossRef
- Butler L, Popkin BM, Poti JM. Associations of alcoholic beverage consumption with dietary intake, waist circumference, and body mass index in US adults: National Health and Nutrition Examination Survey 2003–2012. J Acad Nutr Diet. 2018;118(3):409–420.e3. PubMed CrossRef
- Booranasuksakul U, Singhato A, Rueangsri N, et al. Association between alcohol consumption and body mass index in university students. Asian Pac Isl Nurs J. 2019;4(1):57–65. PubMed CrossRef
- Shen Z, Munker S, Wang C, et al. Association between alcohol intake, overweight, and serum lipid levels and the risk analysis associated with the development of dyslipidemia. J Clin Lipidol. 2014;8(3):273–278. PubMed CrossRef
- Kim J, Chu SK, Kim K, et al. Alcohol use behaviors and risk of metabolic syndrome in South Korean middle-aged men. BMC Public Health. 2011;11(1):489. PubMed CrossRef
- Tolstrup JS, Halkjaer J, Heitmann BL, et al. Alcohol drinking frequency in relation to subsequent changes in waist circumference. Am J Clin Nutr. 2008;87(4):957–963. PubMed CrossRef
- Thomson CA, Wertheim BC, Hingle M, et al. Alcohol consumption and body weight change in postmenopausal women: results from the Women’s Health Initiative. Int J Obes. 2012;36(9):1158–1164. PubMed CrossRef
- Pajari M, Pietiläinen KH, Kaprio J, et al. The effect of alcohol consumption on later obesity in early adulthood: a population-based longitudinal study. Alcohol Alcohol. 2010;45(2):173–179. PubMed CrossRef
- Arabshahi S, Lahmann PH, Williams GM, et al. Predictors of change in weight and waist circumference: 15-year longitudinal study in Australian adults. Eur J Clin Nutr. 2014;68(3):309–315. PubMed CrossRef
- Rahimi N, Gozashti MH, Najafipour H, et al. Potential effect of opium consumption on controlling diabetes and some cardiovascular risk factors in diabetic patients. Addict Health. 2014;6(1-2):1–6. PubMed
- Hosseini SK, Masoudkabir F, Vasheghani-Farahani A, et al. Opium consumption and coronary atherosclerosis in diabetic patients: a propensity score-matched study. Planta Med. 2011;77(17):1870–1875. PubMed CrossRef
- Asgary S, Sarrafzadegan N, Naderi G-A, et al. Effect of opium addiction on new and traditional cardiovascular risk factors: do duration of addiction and route of administration matter? Lipids Health Dis. 2008;7(1):42. PubMed CrossRef
- Azarasa M, Azarfarin R, Changizi A, et al. Substance use among Iranian cardiac surgery patients and its effects on short-term outcome. Anesth Analg. 2009;109(5):1553–1559. PubMed CrossRef
- Azod L, Rashidi M, Afkhami-Ardekani M, et al. Effect of opium addiction on diabetes. Am J Drug Alcohol Abuse. 2008;34(4):383–388. PubMed CrossRef
- Kouros D, Tahereh H, Mohammadreza A, et al. Opium and heroin alter biochemical parameters of human’s serum. Am J Drug Alcohol Abuse. 2010;36(3):135–139. PubMed CrossRef
- Najafipour H, Beik A. The impact of opium consumption on blood glucose, serum lipids and blood pressure, and related mechanisms. Front Physiol. 2016;7:436. PubMed
- Karam GA, Reisi M, Kaseb AA, et al. Effects of opium addiction on some serum factors in addicts with non-insulin-dependent diabetes mellitus. Addict Biol. 2004;9(1):53–58. PubMed CrossRef
- Tan XJ, Jiao GP, Ren YJ, et al. Relationship between smoking and dyslipidemia in western Chinese elderly males. J Clin Lab Anal. 2008;22(3):159–163. PubMed CrossRef
- Virdis A, Giannarelli C, Fritsch Neves M, et al. Cigarette smoking and hypertension. Curr Pharm Des. 2010;16(23):2518–2525. PubMed CrossRef
- Bermudez V, Olivar LC, Torres W, et al. Cigarette smoking and metabolic syndrome components: a cross-sectional study from Maracaibo City, Venezuela. F1000 Res. 2018;7:565. PubMed CrossRef
- Bathla M, Singh M, Anjum S, et al. Metabolic syndrome in drug naïve patients with substance use disorder. Diabetes Metab Syndr. 2017;11(3):167–171. PubMed CrossRef
- Kahl KG, Greggersen W, Schweiger U, et al. Prevalence of the metabolic syndrome in men and women with alcohol dependence: results from a cross-sectional study during behavioral treatment in a controlled environment. Addiction. 2010;105(11):1921–1927. PubMed CrossRef
- French MT, Norton EC, Fang H, et al. Alcohol consumption and body weight. Health Econ. 2010;19(7):814–832. PubMed CrossRef
- Oliveros E, Somers VK, Sochor O, et al. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014;56(4):426–433. PubMed CrossRef
- Rajs J, Petersson A, Thiblin I, et al. Nutritional status of deceased illicit drug addicts in Stockholm, Sweden: a longitudinal medicolegal study. J Forensic Sci. 2004;49(2):320–329. PubMed CrossRef
- Pucci G, Alcidi R, Tap L, et al. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res. 2017;120:34–42. PubMed CrossRef
- Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006;95(3):136–147. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top