Letter to the Editor: Mpox is a zoonotic viral disease similar to smallpox that is transmitted through close contact with an infected person, animal, or object contaminated with the virus.1,2 It occurs primarily in tropical regions of central and West Africa. However, since May 2022, cases of Mpox have been reported from regions where the disease is not endemic. Confirmed cases with travel history in Europe and North America were reported. In addition, Mpox cases and clusters have been reported concurrently in non-endemic and endemic countries spanning a wide geographic area.3 At the time of this writing, since September 23, 2022, there have been a total of 65,415 cases and 26 deaths worldwide due to the recent global outbreak.4,5
Although Mpox is usually self-limited, with symptoms lasting 2–4 weeks, a study suggested that 13% of patients diagnosed with Mpox required hospitalization.3,4 Tecovirimat, an antiviral that works by inhibiting p37 (a protein involved in the release of the enveloped virus, dissemination, and virulence) that has been approved by the US Food and Drug Administration (FDA) to treat smallpox, has been shown to have activity against Mpox in vitro and has a favorable clinical safety profile in healthy volunteers.6,7 Cidofovir is another antiviral medication that has been shown to be effective against orthopoxviruses in vitro and animal studies.8 Sufficient data are unavailable on the safety and effectiveness of tecovirimat or cidofovir in treating people with Mpox. The FDA has approved tecovirimat for Mpox in emergencies for populations who meet specific criteria. Under the expanded access of investigational new drug (EA-IND) protocol, it has been made available for prescription in patients with Mpox who have or are at high risk of severe disease.7,9
Clinically significant side effects of tecovirimat have been reported in < 2% of subjects exposed to the medication.7,8 Psychiatric side effects of tecovirimat include depression, dysphoria, irritability, and panic attack. Given the high incidence and spread of Mpox, it is crucial to discuss the psychopharmacologic interactions of experimental drugs in the treatment of widespread Mpox: (1) tecovirimat and (2) cidofovir. This report reviews tecovirimat and cidofovir’s pharmacologic interactions with psychotropic medications/antidepressants, antipsychotics, mood stabilizers, and sedatives.7,9,10
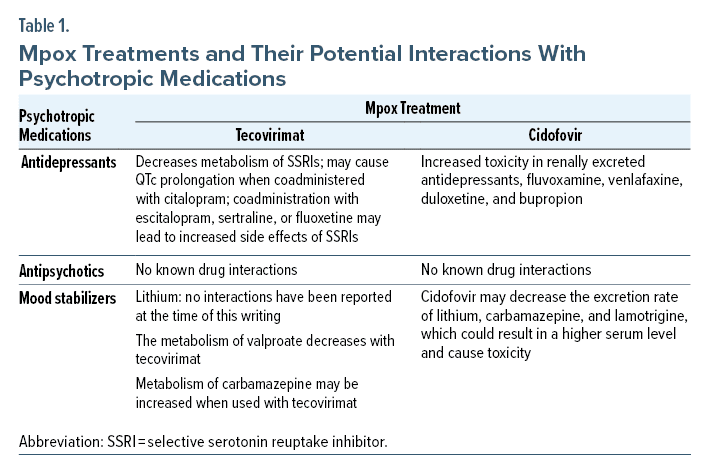
Antidepressants
Tecovirimat undergoes hydrolysis by UGT1A1 and 1A4 enzymes. Additionally, it can act as a cytochrome P450 (CYP) 2C19 inhibitor. When coadministered with citalopram, there could be QTc prolongation due to a decrease in the metabolism of citalopram by CYP2C19.11 Similarly, when coadministered with escitalopram, sertraline, or fluoxetine, levels of the drug may increase, leading to increased side effects.12 Cidofovir has been known to cause nephrotoxicity and could impair the metabolism of renally excreted drugs.13 When administered with antidepressants, fluvoxamine, venlafaxine, duloxetine, and bupropion, drug concentrations may increase, leading to increased toxicity.13 There are no known drug interactions with any other antidepressants.
Antipsychotics
Tecovirimat is mainly metabolized by hydrolysis and glucuronidation by UGT1A1 and UGT1A4. The hepatic route and biotransformation metabolize most antipsychotics. Most of the atypical antipsychotics are metabolized by the CYP substrate system. Clozapine and olanzapine are metabolized by the CYPA12 pathway, risperidone mainly by the CYP2D6, and quetiapine and ziprasidone mainly by the CYP3A4 pathways. There are no known interactions between the novel drug tecovirimat, which is widely used for Mpox, and the typical or atypical antipsychotic medications.14 Since this drug is still in the experimental stage, there is a need for more data to find conclusive evidence regarding the same.
Mood Stabilizers
Mood stabilizers are some of the most used psychiatric drugs, and it is essential to understand their interactions. They are administered orally and undergo hydrolysis mediated by UGT1A1 and UGT1A4, and none of their metabolites are active.15 Lithium is the most utilized mood stabilizer and is relatively safe to be used with tecovirimat, as no interactions have been reported as of this writing. The metabolism of valproate can be reduced when given with tecovirimat, as valproate undergoes glucuronidation (UGTs 1A6, 1A9, and 2B7) and CYP enzymes (CYP2C9 and CYP2C196) and tecovirimat interact with them.16 Carbamazepine is a CYP3A4 inducer, and tecovirimat is a weak inhibitor of CYP2C8 and CYP2C9 and a weak inducer of CYP3A4. Therefore, the metabolism of carbamazepine can be increased when used with tecovirimat.17 Cidofovir may decrease the excretion rate of lithium, carbamazepine, and lamotrigine, which could result in a higher serum level and cause toxicity. Therefore, serum levels of these anticonvulsants should be checked.18 Brincidofovir is a lipid conjugate prodrug of the acyclic nucleotide analog cidofovir, which the FDA also approves for treating Mpox. Currently, no interaction with mood stabilizers is seen.18
Conclusion
Treatments for Mpox, namely tecovirimat and cidofovir, have documented interactions with psychotropic medications (Table 1). However, since the treatment of Mpox is in the early and experimental stages, observing and documenting the interactions of Mpox treatment medications with psychotropics is crucial. The data to date are insufficient to comment substantially on interactions of tecovirimat and cidofovir with common psychotropic medications: antidepressants, antipsychotics, and mood stabilizers.
Article Information
Published Online: February 15, 2024. https://doi.org/10.4088/PCC.23lr03608
© 2024 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2024;26(1):23lr03608
To Cite: Mansuri Z, Shah B, Yadav G, et al. Drug interactions between psychotropic medications and treatment of Mpox (tecovirimat and cidofovir). Prim Care Companion CNS Disord. 2024;26(1):23lr03608.
Author Affiliations: Department of Psychiatry, Boston Children’s Hospital/Harvard Medical School, Boston, Massachusetts (Mansuri); Department of Psychiatry and Behavioral Science, University of Louisville, Kentucky (Shah); Department of Psychiatry, Texas Tech University Health Science Center at Permian Basin, Midland, Texas (Yadav); Department of Psychiatry, Bronx Care Health System at Icahn School of Medicine at Mount Sinai, New York (Kainth); Department of Psychiatry, Maimonides Medical Center, Brooklyn, New York (Kochhar); Department of Psychiatry, Baptist Health—UAMS Psychiatry Residency Education Program, North Little Rock, Arkansas (Srinivas, Bachu); Department of Pediatrics, Dell Children’s Medical Center, Austin, Texas (Kamil); Department of Psychiatry, Virginia Tech Carilion School of Medicine, Roanoke, Virginia (Reddy). Drs Mansuri and Shah are shared first authors. Drs Bachu and Reddy are shared senior authors.
Corresponding Author: Zeeshan Mansuri, MD, MPH, Boston Children’s Hospital/Harvard Medical School, 300 Longwood Ave, Boston, MA 02115 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
References (18)

- World Health Organization. Mpox (monkeypox). Accessed December 12, 2023. www.who.int/news-room/fact-sheets/detail/monkeypox
- Adler H, Gould S, Hine P, et al; NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153–1162. PubMed CrossRef
- World Health Organization. Mpox (monkeypox) outbreak. Accessed December 12, 2023. https://www.who.int/emergencies/situations/monkeypox-oubreak-2022
- Thornhill JP, Barkati S, Walmsley S, et al; SHARE-net Clinical Group. Monkeypox virus infection in humans across 16 Countries: April-June 2022. N Engl J Med. 2022;387(8):679–691. PubMed CrossRef
- Centers for Disease Control and Prevention. 2022 Monkeypox Outbreak Global Map. Accessed December 12, 2023. https://www.cdc.gov/poxvirus/mpox/response/2022/world-map.html
- Yang G, Pevear DC, Davies MH, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J Virol. 2005;79(20):13139–13149. PubMed CrossRef
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379(1):44–53. PubMed CrossRef
- Centers for Disease Control and Prevention. Treatment Information for Healthcare Professionals. Accessed December 12, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Office of the Commissioner. Expanded Access. US Food and Drug Administration. https://www.fda.gov/news-events/public-health-focus/expanded-access
- Tecovirimat. Summary of Product Characteristics. Accessed December 12, 2023. https://www.ema.europa.eu/en/documents/product-information/tecovirimat-siga-epar-product-information_en.pdf
- Deshmukh A, Ulveling K, Alla V, et al. Prolonged QTc interval and torsades de pointes induced by citalopram. Tex Heart Inst J. 2012;39(1):68–70. PubMed
- Mössner LD, Schmitz A, Theurillat R, et al. Inhibition of cytochrome P450 enzymes involved in ketamine metabolism by use of liver microsomes and specific cytochrome P450 enzymes from horses, dogs, and humans. Am J Vet Res. 2011;72(11):1505–1513. PubMed CrossRef
- Bonate PL, Reith K, Weir S. Drug interactions at the renal level. Implications for drug development. Clin Pharmacokinet. 1998;34(5):375–404. PubMed CrossRef
- Urichuk L, Prior TI, Dursun S, et al. Metabolism of atypical antipsychotics: involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr Drug Metab. 2008;9(5):410–418. PubMed CrossRef
- FDA Approved Drug Products: TPOXX (tecovirimat) capsules, for oral use or injection, for intravenous use; Summary of Product Characteristics: Tecovirimat (tecovirimat) oral capsules
- Tecovirimat. Accessed December 12, 2023. https://go.drugbank.com/drugs/DB12020
- US Food and Drug Administration. Drugs@FDA Data Files. Accessed December 12, 2023. https://www.fda.gov/drugs/drug-approvals-and-databases/drugsfda-data-files
- Delaune D, Iseni F. Drug development against smallpox: present and future. Antimicrob Agents Chemother. 2020;64(4):e01683. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top