Introduction: Bipolar disorder is a complex mood disorder characterized by a chronic and subtle course of fluctuating manic/hypomanic and depressive symptoms. Cariprazine, a dopamine D3-preferring D3/D2 receptor partial agonist with serotonin 5-HT1A receptor partial agonist and serotonin 5-HT2A antagonist properties, is approved to treat manic and depressive episodes of bipolar disorder. Post hoc analyses evaluated efficacy across symptoms in bipolar depression.
Methods: Pooled data were analyzed from 3 phase 2 or 3, randomized, double-blind, placebo-controlled studies of adults with bipolar disorder and a major depressive episode. Mean change from baseline to week 6 in Montgomery-Asberg Depression Rating Scale (MADRS) total score and individual item scores were analyzed in individual dose groups (1.5 mg/d, 3 mg/d) and overall cariprazine (1.5-3 mg/d). Pooled safety was evaluated via adverse events.
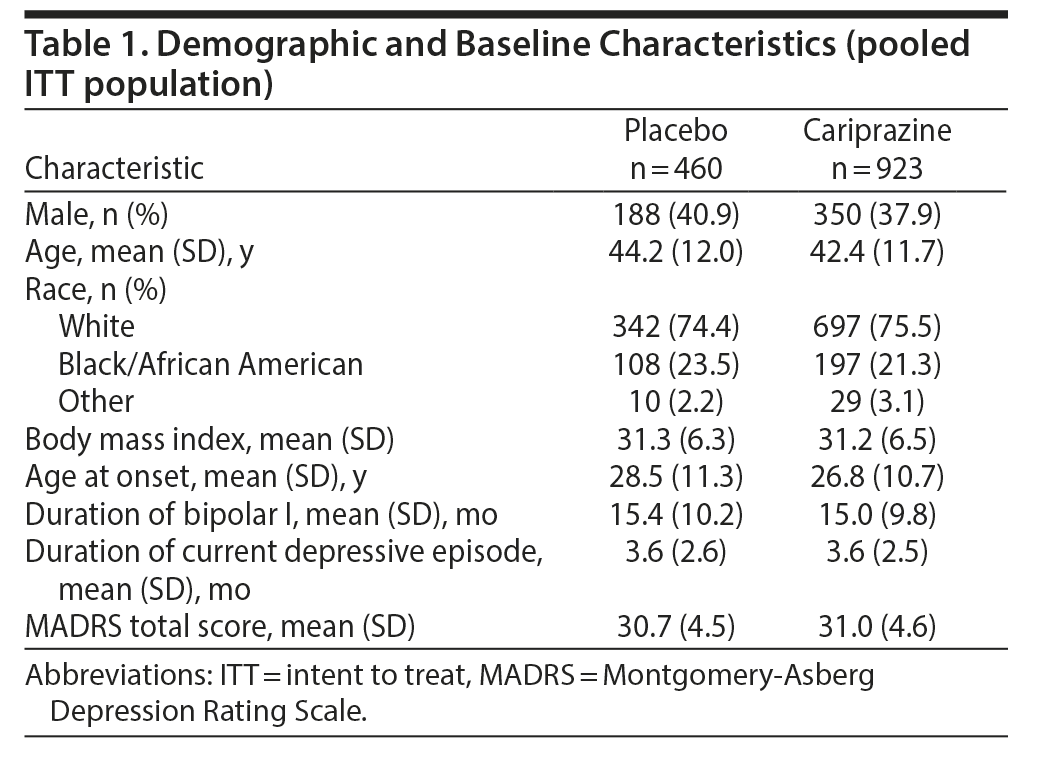
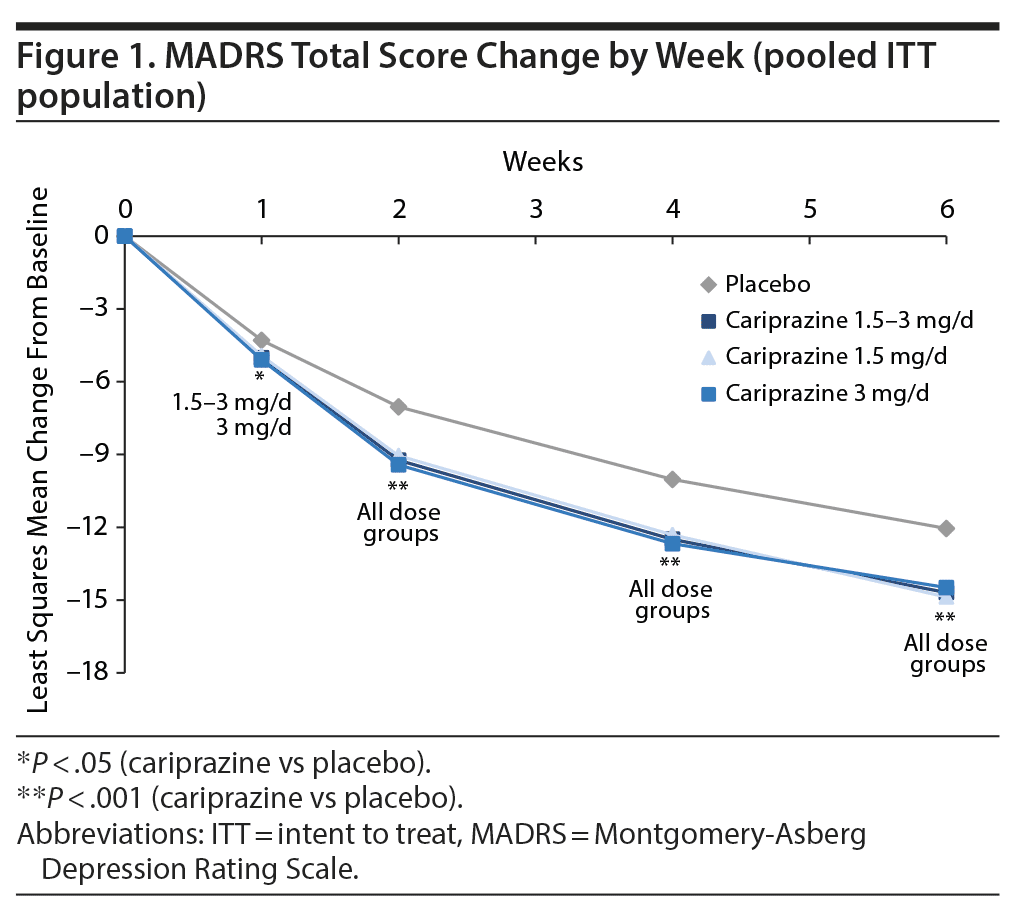
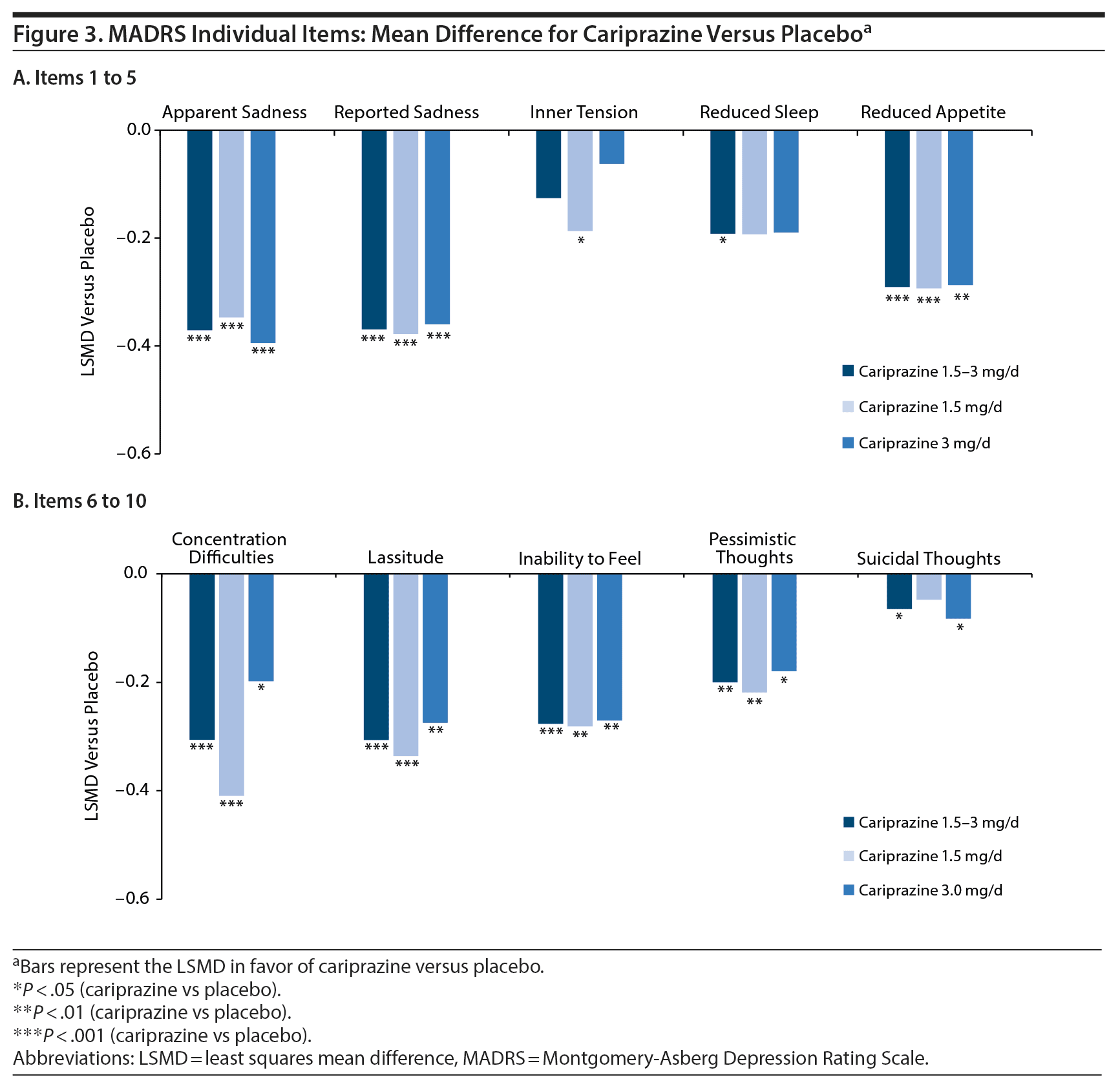
Results: A significantly greater difference in mean change from baseline in MADRS total score was seen for each cariprazine dose group versus placebo (least squares mean difference vs placebo: 1.5-3 mg/d = −2.6, 1.5 mg/d = −2.8, 3 mg/d = −2.4) (P < .001 all). Significant differences versus placebo were seen on all individual MADRS items except inner tension for the overall cariprazine group (P < .05). Cariprazine was generally well tolerated.
Conclusions: Cariprazine demonstrated broad efficacy across symptoms of depression in bipolar disorder. In previous post hoc analyses, cariprazine also demonstrated broad efficacy across manic symptoms, suggesting that it is effective across the wide range of symptoms on the bipolar spectrum. A 1.5-mg/d starting dose and slow titration resulted in lower rates of some adverse events in the bipolar depression studies versus the mania studies.
Trial Registration: ClinicalTrials.gov identifiers: NCT01396447, NCT02670538, NCT02670551
aheadofprint=’article’;
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Broad Efficacy of Cariprazine on Depressive Symptoms in Bipolar Disorder and the Clinical Implications
ABSTRACT
Introduction: Bipolar disorder is a complex mood disorder characterized by a chronic and subtle course of fluctuating manic/hypomanic and depressive symptoms. Cariprazine, a dopamine D3-preferring D3/D2 receptor partial agonist with serotonin 5-HT1A receptor partial agonist and serotonin 5-HT2A antagonist properties, is approved to treat manic and depressive episodes of bipolar disorder. Post hoc analyses evaluated efficacy across symptoms in bipolar depression.
Methods: Pooled data were analyzed from 3 phase 2 or 3, randomized, double-blind, placebo-controlled studies of adults with bipolar disorder and a major depressive episode. Mean change from baseline to week 6 in Montgomery-Asberg Depression Rating Scale (MADRS) total score and individual item scores were analyzed in individual dose groups (1.5 mg/d, 3 mg/d) and overall cariprazine (1.5-3 mg/d). Pooled safety was evaluated via adverse events.
Results: A significantly greater difference in mean change from baseline in MADRS total score was seen for each cariprazine dose group versus placebo (least squares mean difference vs placebo: 1.5-3 mg/d = −2.6, 1.5 mg/d = −2.8, 3 mg/d = −2.4) (P < .001 all). Significant differences versus placebo were seen on all individual MADRS items except inner tension for the overall cariprazine group (P < .05). Cariprazine was generally well tolerated.
Conclusions: Cariprazine demonstrated broad efficacy across symptoms of depression in bipolar disorder. In previous post hoc analyses, cariprazine also demonstrated broad efficacy across manic symptoms, suggesting that it is effective across the wide range of symptoms on the bipolar spectrum. A 1.5-mg/d starting dose and slow titration resulted in lower rates of some adverse events in the bipolar depression studies versus the mania studies.
Trial Registration: ClinicalTrials.gov identifiers: NCT01396447, NCT02670538, NCT02670551
Prim Care Companion CNS Disord 2020;22(5):20m02611
To cite: Yatham LN, Vieta E, McIntyre RS, et al. Broad efficacy of cariprazine on depressive symptoms in bipolar disorder and the clinical implications. Prim Care Companion CNS Disord. 2020;22(5):20m02611.
To share: https://doi.org/10.4088/PCC.20m02611
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of British Columbia, Vancouver, British Columbia, Canada
bDepartment of Psychiatry and Psychology, Hospital Clinic, University of Barcelona, IDIBAPS, CIBERSAM, Barcelona, Spain
cMood Disorders Psychopharmacology Unit, University Health Network, Toronto, Ontario, Canada
dDepartment of Psychiatry, Texas Tech University School of Medicine-Permian Basin, Midland, Texas
eMedical Affairs, AbbVie, Madison, New Jersey
fClinical Development, AbbVie, Madison, New Jersey
*Corresponding author: Lakshmi N. Yatham, MBBS, FRCPC, MBA, University Hospital—Detwiller Pavilion 2883, 2255 Wesbrook Mall, Vancouver, BC, V6T 2A1, Canada ([email protected]).
Bipolar disorder is a complex and disabling mood disorder that is characterized by a chronic and subtle course of fluctuating manic, hypomanic, and depressive symptoms.1,2 On the bipolar spectrum, bipolar depression is the leading cause of morbidity in patients with bipolar disorder,3 with major depressive disorder (MDD), dysthymia, and dysphoric mixed states accounting for a far greater percentage of time spent unwell than manic symptoms.4-9 For example, a systematic review10 of studies of long-term course in clinically treated patients with bipolar I disorder concluded that depressive symptoms account for approximately 70% of the time spent unwell with the disorder. The excess burden of depressive symptoms compared with manic symptoms in bipolar disorder is underscored by greater long-term dysfunction, worse psychosocial impairment, more lost work productivity, worse cognitive impairment, and diminished quality of life.11 Comorbidities are common,12 adding to the symptom burden and complicating the clinical picture. Effective treatment across the spectrum of bipolar symptoms is a necessity for patients with this complex disorder.
Although diagnosing bipolar disorder may not be challenging if a patient presents with blatant manic symptoms, 50% of individuals with bipolar disorder seek treatment during a depressive episode.13 As such, misdiagnosis is common, especially if a patient is seen early in the course of illness and corroborating information that would establish a history of hypomania or mania is lacking.3 Unipolar depression is the initial misdiagnosis for 60% of patients with bipolar disorder,14 although approximately 20% of patients initially diagnosed with MDD subsequently experience a manic or hypomanic episode, resulting in a revised diagnosis of bipolar I or II disorder.15 Misdiagnosis as MDD is especially problematic given the progressive nature of bipolar disorder, the potential for mistreatment with antidepressants, and delayed initiation of effective treatment.16 Antidepressant monotherapy is not recommended for bipolar depression because of the lack of evidence for efficacy and abiding safety concerns, including the risk for rapid cycling or treatment-emergent mania.16,17
Approximately half of all patients with mental illness are treated in primary care.18 To promote timely and accurate diagnosis and effective treatment of bipolar disorder, it is important that all clinicians recognize when symptoms such as mania, hypomania, depression, subsyndromal depression, or anxiety are part of the bipolar spectrum. Although several medications are US Food and Drug Administration approved to treat bipolar mania, only cariprazine, quetiapine (immediate and extended release), lurasidone, and combination fluoxetine/olanzapine are approved to treat bipolar depression. Of these agents, only cariprazine and quetiapine are approved to treat both manic and depressive symptoms. Cariprazine is a dopamine D3-preferring D3/D2 receptor partial agonist with serotonin 5-HT1A receptor partial agonist and serotonin 5-HT2A antagonist properties. As demonstrated in preclinical studies, this unique D3 receptor affinity may mediate antianhedonic, procognitive, and antidepressant-like effects and improve social memory deficits,19-22 suggesting that it may have broad utility to treat a range of symptoms in bipolar disorder.
The efficacy, safety, and tolerability of cariprazine (3-12 mg/d) in manic or mixed episodes of bipolar I disorder were established in 3 randomized clinical trials23-25; the recommended dose range in bipolar mania is 3-6 mg/d.26 The indication for bipolar depression was based on efficacy and safety outcomes in 3 subsequent randomized studies27-29 in patients with bipolar I disorder who were experiencing a major depressive episode. In these trials, 3 fixed cariprazine doses (0.75 mg [1 study], 1.5 mg, and 3 mg [each study]) were evaluated on the primary endpoint, change from baseline to week 6 in Montgomery-Asberg Depression Rating Scale (MADRS)30 total score. In each trial, a statistically significant difference was seen in favor of cariprazine 1.5 mg/d versus placebo, while cariprazine 3 mg separated from placebo in 1 study (RGH-MD-54) and was numerically better in 2 studies (RGH-MD-56 and -53); 0.75 mg did not separate from placebo. The recommended cariprazine dose in bipolar depression is 1.5 mg/d or 3 mg/d.26
To explore the efficacy of cariprazine across the debilitating symptoms of bipolar depression, we conducted post hoc analyses of pooled data from the 3 clinical trials in bipolar depression. For these findings to have a clinically useful context for primary care physicians, the efficacy and safety profile of cariprazine across mania and other symptoms of bipolar disorder is also presented so treatment decisions can be made in the best interest of the whole patient.

- Cariprazine is 1 of only 2 treatments that has demonstrated efficacy in both mania and depression associated with bipolar I disorder and is approved for both of these indications.
- Cariprazine demonstrated efficacy across the symptoms of depression in patients with bipolar I disorder.
- Cariprazine is easy to use, as the starting dose of 1.5 mg is the therapeutic dose, and it is generally well tolerated.
METHODS
Study Design and Patients
Data from 3 bipolar depression studies (RGH-MD-56 [NCT01396447, July 2011-January 2014], RGH-MD-53 [NCT02670538, March 2016-January 2018], and RGH-MD-54 [NCT02670538, March 2016-July 2017]) were pooled for analyses. The studies were similarly designed phase 2 or 3, randomized, placebo-controlled, double-blind, multicenter, parallel-group, fixed-dose studies of adult patients with bipolar disorder experiencing a major depressive episode; detailed methods were published previously.27-29 Briefly, all studies consisted of a screening period (up to 14 days) followed by double-blind treatment and a 1-week safety follow-up period; the double-blind period was 8 weeks in RGH-MD-56 and 6 weeks in RGH-MD-53 and RGH-MD-54, although the primary endpoint was week 6 in all studies. In RGH-MD-56, patients were randomized (1:1:1:1) to placebo, cariprazine 0.75 mg/d, cariprazine 1.5 mg/d, or cariprazine 3 mg/d; patients randomized to cariprazine were initiated on 0.5 mg/d and uptitrated to target doses of 0.75 mg/d, 1.5 mg/d, or 3 mg/d by day 15, after which the dose was fixed. In RGH-MD-53 and RGH-MD-54, patients were randomized (1:1:1) to placebo, cariprazine 1.5 mg/d, or cariprazine 3 mg/d; cariprazine-treated patients were initiated at a therapeutic 1.5-mg/d dose and patients in the 3-mg/d group were uptitrated to the target dose on day 15. All patients gave informed written consent after study procedures and the potential side effects of the intervention were fully explained; study protocols were approved by the institutional review board (US centers) or ethics committee/government agency (non-US centers).
Male or female outpatients (18-65 years of age) with a diagnosis of bipolar I disorder and a current major depressive episode according to Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria (DSM-IV-TR31 in RGH-MD-56; DSM-532 in RGH-MD-53 and RGH-MD-54) participated in the studies. Clinical inclusion criteria required that patients have a 17-item Hamilton Depression Rating Scale (HDRS-17)33 total score ≥ 20 and an item 1 score ≥ 2. Exclusion criteria included a score ≥ 12 (or > 10 in RGH-MD-56) on the Young Mania Rating Scale (YMRS),34 DSM axis I diagnosis other than bipolar I disorder, alcohol- or substance-related disorders (within 6 months), and risk for suicide (investigator judged or rating scale assessment). Patients with nonresponse to 2 or more treatment trials of adequate dose and duration with an approved bipolar depression agent in the current depressive episode were also ineligible.
Post Hoc Analyses
Data from the 3 bipolar depression studies were pooled and analyzed in individual dose groups (1.5 mg/d or 3 mg/d) and overall cariprazine (pooled 1.5 and 3 mg/d); the 0.75-mg/d dose was not included in these analyses because it is not within the recommended dose range for cariprazine. Post hoc outcomes of interest were mean change from baseline to the end of week 6 in MADRS total score and individual item scores. Pooled safety and tolerability in the recommended dose range for bipolar depression from these 3 studies were also investigated via reports of adverse events (AEs).
Statistical Analyses
MADRS outcomes were based on the pooled intent-to-treat (ITT) population (all patients in the safety population with a baseline and ≥ 1 postbaseline MADRS total score assessment). Change from baseline to week 6 in MADRS total and individual item scores was analyzed using a mixed-effects model for repeated measures (MMRM) with study, treatment group, visit, and treatment group-by-visit as factors and baseline MADRS scores and baseline-by-visit interaction as covariates. P values were not adjusted for multiple comparisons; all statistical tests were 2-sided at the 5% significance level. AEs were analyzed using descriptive statistics; no inferential statistics were performed for this safety parameter.
RESULTS
Patient Disposition and Demographics
The pooled ITT population comprised 1,383 patients (placebo = 460, cariprazine = 923 [1.5 mg/d = 461, 3 mg/d = 462]). In each constituent study, baseline and demographic characteristics were similar between groups; characteristics of the pooled placebo and cariprazine groups are presented in Table 1. Mean baseline MADRS total scores in the placebo and cariprazine groups indicated a population with moderate depression.35
MADRS Total Score
Improvement in depressive symptoms was demonstrated by significantly greater mean change from baseline in MADRS total score for each cariprazine dose group versus placebo at week 6; the least squares mean difference (LSMD) in favor of cariprazine versus placebo was −2.6 for 1.5-3 mg/d, −2.8 for 1.5 mg/d, and −2.4 for 3 mg/d. A significant difference in favor of cariprazine versus placebo was seen as early as week 1 for overall cariprazine and cariprazine 3 mg/d and week 2 for cariprazine 1.5 mg/d; significant differences versus placebo persisted from the point of separation through week 6 for all groups (Figure 1).
MADRS Individual Items
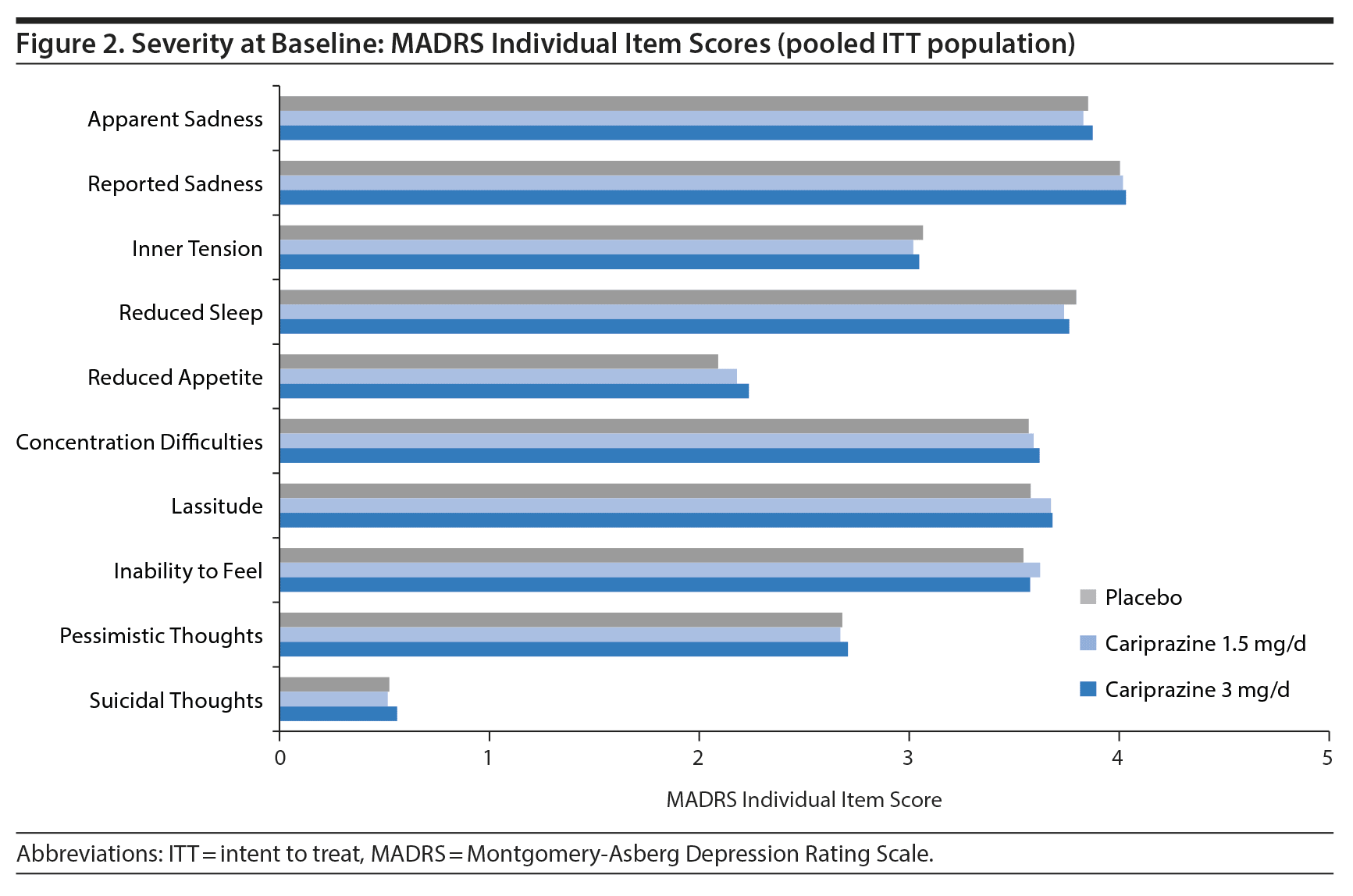
To investigate efficacy across individual depressive symptoms, the single items of the MADRS were analyzed. The severity of mean baseline scores on all individual MADRS items except suicidal thoughts ranged from 2 (mild symptoms) to 4 (moderate symptoms) (Figure 2).
Improvement across depressive symptoms was shown by statistically significant LSMDs versus placebo in mean change from baseline on all individual MADRS items except inner tension in the combined 1.5- to 3-mg/d dose group and on most items in the 1.5-mg and 3-mg individual dose groups (Figure 3A and Figure 3B). Mean changes in suicidal thoughts were small, but the differences were still significant in favor of cariprazine for the overall and 3-mg/d groups; small mean changes are most likely due to low baseline scores on this item.
Safety and Tolerability in Bipolar Depression
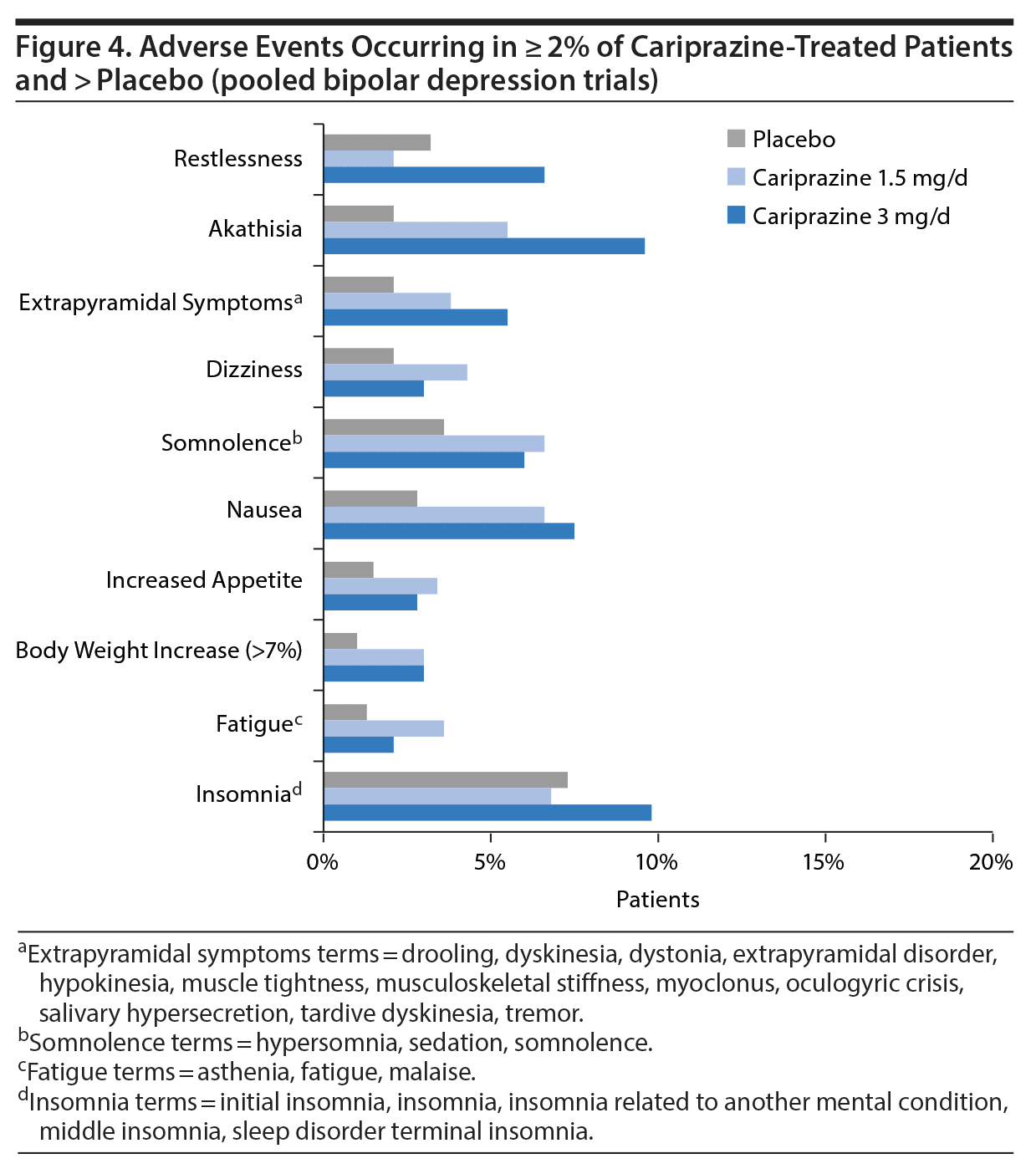
In a pooled post hoc analysis of safety data from the clinical studies of cariprazine in patients with bipolar depression (RGH-MD-53, -54, and -56), recommended doses were generally well tolerated. Overall, few patients discontinued from the studies due to AEs, with similar rates for cariprazine (6%) and placebo (5%). The only AEs that occurred in ≥ 5% of patients and at least twice the rate of placebo were nausea, akathisia, restlessness, and extrapyramidal symptoms. Akathisia was the most common treatment-emergent AE, but it rarely resulted in discontinuation from a study (1.5 mg/d = 1%, 3 mg/d = 2%). The most common AEs occurring in more cariprazine- than placebo-treated patients are presented in Figure 4. Cariprazine had a neutral metabolic profile, with similar proportions of cariprazine- and placebo-treated patients having shifts in fasting total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides. Weight increase was minimal (mean change < 1 kg) in both dose groups.
DISCUSSION
As the only dopamine antagonist/partial agonist approved to treat both bipolar mania and depression, cariprazine occupies a unique place in the bipolar disorder treatment armamentarium. Although several agents are approved to treat symptoms of bipolar mania, few agents have proven efficacy in treating acute bipolar depression. For example, in 23 clinical trials that evaluated the efficacy of 9 different agents in monotherapy, only olanzapine (2 trials), lurasidone (1 trial), quetiapine (5 trials), and cariprazine (3 trials) were more effective than placebo in improving depressive symptoms in patients with bipolar depression.36 Our post hoc analyses of pooled data from these same 3 cariprazine trials extended its evidence for efficacy in bipolar depression by demonstrating improvement across a wide range of individual depressive symptoms in patients with bipolar I disorder. Significant differences versus placebo were seen on all MADRS individual items except inner tension for the cariprazine 1.5- to 3-mg/d group. Further, since sadness, lassitude, and inability to feel are key symptoms that clinicians regularly encounter in primary care settings and ones that they may struggle to assess in the context of bipolar disorder, it is noteworthy that cariprazine had strong effects on these individual MADRS items, suggesting it as a treatment option that could help address problematic symptoms in the clinic.
Beyond efficacy in bipolar depression, cariprazine has also demonstrated robust efficacy in bipolar mania. In a post hoc analysis of data from the 3 positive clinical trials in patients with acute manic or mixed episodes associated with bipolar I disorder,23-25 the difference in change from baseline to week 3 in Young Mania Rating Scale (YMRS)34 total score was statistically significant in favor of a cariprazine versus placebo at all assessments from visit 1 on day 4 to the last visit on day 21 (P < .001).37 In subsequent investigations of broad efficacy across the individual symptoms of mania based on data from these same 3 studies, equally robust efficacy was demonstrated by statistically significant differences in favor of cariprazine over placebo in mean change from baseline to week 3 on all 11 individual items of the YMRS (P < .001).38 Together with the results from our bipolar depression analyses, these post hoc outcomes suggest that cariprazine has strong efficacy across both overall and individual manic and depressive symptoms, which is an important characteristic for a bipolar treatment given the changeable and wide-ranging symptoms that are typical of this disorder.
Symptoms other than depression and mania are also common and burdensome in bipolar disorder. Anxiety, which may occur as another symptom or as a comorbid condition of bipolar disorder, is frequently associated with increasing symptom severity, frequency of episodes, higher suicide rates, and decreasing response to therapy in bipolar disorder.39 In the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study, 51% of patients had at least 1 type of lifetime anxiety disorder.40 The effect of cariprazine on anxiety symptoms was explored in 2 of the bipolar depression studies via the Hamilton Anxiety Rating Scale (HARS),41 which was included as an additional efficacy measure. In both studies,28,29 the mean difference in change from baseline to week 6 in HARS total scores was statistically significant in favor cariprazine 1.5 mg/d versus placebo (P < .05). While this finding suggests that anxiety symptoms in patients with bipolar depression may improve with cariprazine treatment, especially at the 1.5-mg/d dose, additional analyses are warranted to further explore this common and debilitating aspect of bipolar disorder.
Adding to the challenges of treating bipolar disorder, there is increasing evidence that manic or depressive episodes with mixed features are common, presenting in 25%-35% of all mood episodes.42 Mixed episodes are associated with worse clinical characteristics including earlier age at onset, increased frequency of psychotic symptoms, higher risk of suicide, higher rates of comorbidities including substance use, longer time to remission, more severe course of illness, and worse prognosis.43 Importantly, post hoc analyses of data from the clinical trials of cariprazine in patients with acute bipolar mania23-25 and bipolar depression27-29 have shown preliminary efficacy for mixed features in both bipolar I mania and depression.44,45 Collectively, post hoc results suggest that cariprazine could benefit patients with bipolar mania or depression by treating symptoms across the bipolar spectrum, including symptoms associated with more complex presentations of bipolar disorder.
Efficacy only represents part of the overall treatment picture for bipolar disorder since good safety and tolerability are important aspects of clinical success. Medication side effects were reported as a factor that is significantly associated with treatment nonadherence in bipolar patients in the United States, a troubling finding since nonadherence in bipolar disorder is common and associated with poor clinical outcomes.46 Many agents used to treat bipolar disorder are associated with clinically significant AEs (eg, weight gain, metabolic issues, extrapyramidal symptoms [EPS], sedation, hyperprolactinemia),47 but different agents have different safety profiles.48 In an analysis of pooled data from the clinical trials in bipolar depression (RGH-MD-53, -54, and -56), approved doses of cariprazine were generally well tolerated and few patients discontinued from the studies due to AEs. Akathisia was the most common treatment-emergent AE, but it resulted in low rates of discontinuation (1%-2%). Cariprazine also had a neutral metabolic profile and caused minimal weight increase (mean change < 1 kg).26
Of additional note, better tolerability was observed in the cariprazine bipolar depression studies versus the bipolar mania studies on several measures including study completion (78% vs 71%), treatment-emergent AEs (60% vs 80%), serious AEs (1% vs 6%), and discontinuations due to AEs (7% vs 12%).37,49 This tolerability advantage is most likely related to lower maximum doses and slower titration used in the bipolar depression studies compared with the bipolar mania studies. Specifically, patients in 2 of the bipolar depression studies were initiated on a therapeutic 1.5-mg/d dose (lower starting doses were used in RGH-MD-56) and patients in the 3-mg/d dose group were not uptitrated from 1.5 mg/d to 3 mg/d until day 15. Conversely, patients in the bipolar mania studies received 1.5 mg on day 1 and 3 mg on day 2, with incremental 3-mg increases to a maximum of 12 mg/d within the first week of treatment. Although efficacy was shown across the 3- to 12-mg/d dose range in the bipolar mania studies, lower doses were just as effective as higher doses,23 and some side effects occurred at lower rates with lower doses.37 This finding was considered a factor in determining the recommended 3- to 6-mg/d dose range for patients with acute mania.
Limitations of these analyses include their post hoc nature and the lack of an active comparator. The constituent studies were not powered to detect treatment differences in individual symptoms, and as is typical in post hoc evaluations, P values were not adjusted for multiple comparisons, so random chance could have had a role in determining statistically significant differences. Patients with bipolar II disorder, rapid cycling, significant manic symptoms, and most other psychiatric comorbidities were excluded from participation in the constituent studies, limiting the ability to generalize these findings to other populations on the bipolar spectrum.
Deconstructing a mood episode into individual symptom components may provide useful information about symptoms within the fluctuating and changeable environment of bipolar disorder. In these analyses, statistically significant differences versus placebo in change from baseline in MADRS total score and individual items demonstrated the efficacy of cariprazine in treating symptoms across bipolar depression, suggesting that improvement could encompass full syndromal episodes, subsyndromal episodes, and individual residual symptoms. Results from similar individual YMRS item analyses in patients with bipolar mania further established broad efficacy for cariprazine across the wide-ranging symptoms that comprise bipolar disorder. Additionally, safety analyses suggest that starting with the 1.5-mg/d recommended dose and uptitrating slowly may provide a tolerability advantage. Balance is key in managing the capricious symptoms of bipolar disorder—ideal treatment should support mood stability across the spectrum of manic and depressive symptoms while offering patients a tolerable side effect profile. Because treatment with cariprazine offers both broad spectrum efficacy and tolerability benefits, primary and specialty care providers may consider it a good treatment option for their patients with bipolar I depression.
Submitted: March 2, 2020; accepted June 12, 2020.
Published online: September 17, 2020.
Author contributions: Drs Yatham, Vieta, McIntyre, Jain, Patel, and Earley were involved with the study design, analysis, and interpretation of data. All authors contributed to and have approved the final manuscript and are accountable for all aspects of the work, as well as the ability to identify their individual unique contributions and to ensure the integrity of their contributions.
Potential conflicts of interest: Dr Yatham has received research support from or served as a consultant or speaker for Alkermes, AstraZeneca, Bristol-Myers Squibb, the Canadian Psychiatric Foundation, Canadian Institutes of Health Research, Dainippon Sumitomo, Allergan (now AbbVie), GlaxoSmithKline, Johnson & Johnson, Lilly, Lundbeck, NARSAD, Novartis, Otsuka, Pfizer, Servier, the Stanley Foundation, Sunovion, Teva, Valeant, and Wyeth. Dr Vieta has received grants and served as consultant, advisor, or speaker for AB-Biotics, Abbott, Allergan (now AbbVie), Angelini, AstraZeneca, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Farmindustria, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, SAGE, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda, Brain and Behavior Foundation, the Generalitat de Catalunya (PERIS), the Spanish Ministry of Science, Innovation and Universities (CIBERSAM), EU Horizon 2020, and the Stanley Medical Research Institute. Dr McIntyre has received research grant support from Lundbeck, Shire, Otsuka, National Institute of Mental Health, Stanley Medical Research Institute, Canadian Institutes for Health Research, Brain and Behavior Research Foundation, and Chinese National Natural Research Foundation. He has also received speaker/consultant fees from Lundbeck, Pfizer, AstraZeneca, Eli Lilly, Janssen Sunovion, Bausch Health, Takeda, Otsuka, Shire, Allergan (now AbbVie), Purdue, Minerva, and Neurocrine. Dr Jain has received grant funding from or served as a consultant on advisory boards or on speakers’ bureaus for Addrenex, Alkermes, Allergan (now AbbVie), AstraZeneca, Avanir, Forum, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Otsuka, Pamlab, Pfizer, Rhodes, Supernus, Shionogi, Shire, Sunovion, Supernus, Takeda, Teva, and Tris. Mr Patel is an employee of AbbVie. Dr Earley is an employee of AbbVie and shareholder of AstraZeneca, Eli Lilly, and AbbVie.
Funding/support: This manuscript was supported by funding from Allergan (prior to its acquisition by AbbVie).
Role of the sponsor: Allergan (prior to its acquisition by AbbVie) supplied materials and participated in formulating the outline of the studies but had no role in selection or interpretation of the evidence. The authors had full control of the content and approved the final version.
Previous presentation: Poster presented at the Annual Meeting of the American Society of Clinical Psychopharmacology; May 28-31, 2019; Scottsdale, Arizona and the 15th Annual NEI Congress: Neuroscience Education Institute; November 7-10, 2019; Colorado Springs, Colorado.
Acknowledgments: Statistical analyses were provided by Yan Zhong, PhD, of AbbVie. Writing and editorial assistance were provided by Carol Brown, MS, ELS, of Prescott Medical Communications Group, Chicago, Illinois, a contractor of AbbVie. Dr Zhong and Ms Brown report no conflicts of interest related to the subject of this article.
Additional information: Data inquiries can be submitted at https://www.allerganclinicaltrials.com/en/patient-data/.
REFERENCES
1.Grande I, Berk M, Birmaher B, et al. Bipolar disorder. Lancet. 2016;387(10027):1561-1572. PubMed CrossRef
2.Leboyer M, Kupfer DJ. Bipolar disorder: new perspectives in health care and prevention. J Clin Psychiatry. 2010;71(12):1689-1695. PubMed CrossRef
3.Baldessarini RJ, Vieta E, Calabrese JR, et al. Bipolar depression: overview and commentary. Harv Rev Psychiatry. 2010;18(3):143-157. PubMed CrossRef
4.Baldessarini RJ, Salvatore P, Khalsa HM, et al. Morbidity in 303 first-episode bipolar I disorder patients. Bipolar Disord. 2010;12(3):264-270. PubMed CrossRef
5.Joffe RT, MacQueen GM, Marriott M, et al. A prospective, longitudinal study of percentage of time spent ill in patients with bipolar I or bipolar II disorders. Bipolar Disord. 2004;6(1):62-66. PubMed CrossRef
6.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537. PubMed CrossRef
7.Kupka RW, Altshuler LL, Nolen WA, et al. Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord. 2007;9(5):531-535. PubMed CrossRef
8.Paykel ES, Abbott R, Morriss R, et al. Sub-syndromal and syndromal symptoms in the longitudinal course of bipolar disorder. Br J Psychiatry. 2006;189(2):118-123. PubMed CrossRef
9.Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry. 2003;64(6):680-690, quiz 738-739. PubMed CrossRef
10.Forte A, Baldessarini RJ, Tondo L, et al. Long-term morbidity in bipolar-I, bipolar-II, and unipolar major depressive disorders. J Affect Disord. 2015;178:71-78. PubMed CrossRef
11.Miller S, Dell’ Osso B, Ketter TA. The prevalence and burden of bipolar depression. J Affect Disord. 2014;169(suppl 1):S3-S11. PubMed CrossRef
12.Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8. PubMed CrossRef
13.Mitchell PB, Goodwin GM, Johnson GF, et al. Diagnostic guidelines for bipolar depression: a probabilistic approach. Bipolar Disord. 2008;10(1 pt 2):144-152. PubMed CrossRef
14.Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? results of the National Depressive and Manic-Depressive Association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003;64(2):161-174. PubMed CrossRef
15.Fiedorowicz JG, Endicott J, Leon AC, et al. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry. 2011;168(1):40-48. PubMed CrossRef
16.Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170. PubMed CrossRef
17.Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant-emergent mania in bipolar depression: a systematic review and meta-analysis. Bipolar Disord. 2018;20(3):195-227. PubMed CrossRef
18.Lewis FT, Kass E, Klein RM. An overview of primary care assessment and management of bipolar disorder. J Am Osteopath Assoc. 2004;104(suppl 6):S2-S8. PubMed
19.Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796. PubMed CrossRef
20.Neill JC, Grayson B, Kiss B, et al. Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur Neuropsychopharmacol. 2016;26(1):3-14. PubMed CrossRef
21.Watson DJG, King MV, Gyertyán I, et al. The dopamine D3-preferring D2/D3 dopamine receptor partial agonist, cariprazine, reverses behavioural changes in a rat neurodevelopmental model for schizophrenia. Eur Neuropsychopharmacol. 2016;26(2):208-224. PubMed CrossRef
22.Zimnisky R, Chang G, Gyertyán I, et al. Cariprazine, a dopamine D(3)-receptor-preferring partial agonist, blocks phencyclidine-induced impairments of working memory, attention set-shifting, and recognition memory in the mouse. Psychopharmacology (Berl). 2013;226(1):91-100. PubMed CrossRef
23.Calabrese JR, Keck PE Jr, Starace A, et al. Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2015;76(3):284-292. PubMed CrossRef
24.Durgam S, Starace A, Li D, et al. The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord. 2015;17(1):63-75. PubMed CrossRef
25.Sachs GS, Greenberg WM, Starace A, et al. Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. J Affect Disord. 2015;174:296-302. PubMed CrossRef
26.Vraylar [package insert]. Madison, NJ: Allergan USA, Inc; 2019.
27.Durgam S, Earley W, Lipschitz A, et al. An 8-week randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of cariprazine in patients with bipolar I depression. Am J Psychiatry. 2016;173(3):271-281. PubMed CrossRef
28.Earley W, Burgess MV, Rekeda L, et al. Cariprazine treatment of bipolar depression: a randomized double-blind placebo-controlled phase 3 study. Am J Psychiatry. 2019;176(6):439-448. PubMed CrossRef
29.Earley WR, Burgess MV, Khan B, et al. Efficacy and safety of cariprazine in bipolar I depression: a double-blind, placebo-controlled phase 3 study. Bipolar Disord. 2020;22(4):372-384. PubMed CrossRef
30.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed CrossRef
31.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
32.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
33.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed CrossRef
34.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429-435. PubMed CrossRef
35.Snaith RP, Harrop FM, Newby DA, et al. Grade scores of the Montgomery-Asberg Depression and the Clinical Anxiety Scales. Br J Psychiatry. 1986;148(5):599-601. PubMed CrossRef
36.Yatham LN, Saraf G, Pinto J. Recent advances in treatment of bipolar depression. Curr Psychiatr. 2019;18(10):S5-S8.
37.Earley W, Durgam S, Lu K, et al. Tolerability of cariprazine in the treatment of acute bipolar I mania: a pooled post hoc analysis of 3 phase II/III studies. J Affect Disord. 2017;215:205-212. PubMed CrossRef
38.Vieta E, Durgam S, Lu K, et al. Effect of cariprazine across the symptoms of mania in bipolar I disorder: analyses of pooled data from phase II/III trials. Eur Neuropsychopharmacol. 2015;25(11):1882-1891. PubMed CrossRef
39.McIntyre R, Katzman M. The role of atypical antipsychotics in bipolar depression and anxiety disorders. Bipolar Disord. 2003;5(suppl 2):20-35. PubMed CrossRef
40.Simon NM, Otto MW, Wisniewski SR, et al. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry. 2004;161(12):2222-2229. PubMed CrossRef
41.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. PubMed CrossRef
42.McIntyre RS, Soczynska JK, Cha DS, et al. The prevalence and illness characteristics of DSM-5-defined “mixed feature specifier” in adults with major depressive disorder and bipolar disorder: results from the International Mood Disorders Collaborative Project. J Affect Disord. 2015;172:259-264. PubMed CrossRef
43.Shim IH, Woo YS, Bahk WM. Prevalence rates and clinical implications of bipolar disorder “with mixed features” as defined by DSM-5. J Affect Disord. 2015;173:120-125. PubMed CrossRef
44.McIntyre RS, Masand PS, Earley W, et al. Cariprazine for the treatment of bipolar mania with mixed features: a post hoc pooled analysis of 3 trials. J Affect Disord. 2019;257:600-606. PubMed CrossRef
45.McIntyre RS, Suppes T, Earley W, et al. Cariprazine efficacy in bipolar I depression with and without concurrent manic symptoms: post hoc analysis of 3 randomized, placebo-controlled studies. CNS Spectr. 2020;25(4):502-510. PubMed CrossRef
46.Baldessarini RJ, Perry R, Pike J. Factors associated with treatment nonadherence among US bipolar disorder patients. Hum Psychopharmacol. 2008;23(2):95-105. PubMed CrossRef
47.Cha DS, McIntyre RS. Treatment-emergent adverse events associated with atypical antipsychotics. Expert Opin Pharmacother. 2012;13(11):1587-1598. PubMed CrossRef
48.Liauw SS, McIntyre RS. Atypical antipsychotic tolerability and switching strategies in bipolar disorder. Expert Opin Pharmacother. 2010;11(17):2827-2837. PubMed CrossRef
49.Earley WR, Burgess M, Rekeda L, et al. A pooled post hoc analysis evaluating the safety and tolerability of cariprazine in bipolar depression. J Affect Disord. 2020;263:386-395. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top