ABSTRACT
Objective: To review the published literature over the last 10 years for the efficacy of electroconvulsive therapy (ECT) in refractory somatization disorder.
Data Sources: A comprehensive evidence search of the published literature in the last 10 years (2010–2020) was conducted using the search terms ECT, electroconvulsive therapy, efficacy, effectiveness, use of ECT, chronic pain, somatoform disorders, somatoform pain, somatic symptom disorder, and somatization disorder. The review was limited to articles written in the English language. Databases searched included PsycInfo, MEDLINE, PubMed, Embase, and Google Scholar. A supplementary citation search was also conducted by analyzing the reference lists of identified sources.
Study Selection: The initial search revealed 31 articles of potential relevance.
Data Extraction: The studies were analyzed by both authors to obtain clinical information relevant to meeting the objectives of the review.
Data Synthesis: Five single case studies, 1 case series, and 1 open trial focusing on the use of ECT in somatization disorder were identified for inclusion in the review. There were no controlled trials or systematic reviews, and the evidence collated was of low quality.
Conclusions: This review indicates that ECT may be an effective treatment option for severe and refractory manifestations of somatization disorder. However, further research is required in the assessment of the efficacy, safety, and tolerability of ECT in somatization disorder.
Prim Care Companion CNS Disord 2021;23(3):20r02807
To cite: Yahya AS, Khawaja S. Electroconvulsive therapy as a treatment for somatization disorder. Prim Care Companion CNS Disord. 2021;23(3):20r02807.
To share: https://doi.org/10.4088/PCC.20r02807
© Copyright 2021 Physicians Postgraduate Press, Inc.
aWaltham Forest Older Adults Mental Health Team, North East London NHS Foundation Trust, Red Oak Lodge, London, United Kingdom
*Corresponding author: Ahmed Saeed Yahya, MBBS, MRCPsych, North East London NHS Foundation Trust, Waltham Forest Older Adults Mental Health Team, Red Oak Lodge, London, E11 4HU, United Kingdom ([email protected]).
Somatization disorders can cause substantial distress and impair quality of life. They are characterized by chronic medically unexplained physical symptoms. Somatization disorders may be enduring and carry similar risks to other serious mental disorders. Somatization disorders can be refractory to psychiatric intervention including optimal combinations of pharmacologic and psychological therapies. A Cochrane review, which investigated the efficacy of antidepressants and antipsychotics in treating somatoform disorders, found low-quality evidence for these treatments.1,2 Many patients are prescribed multiple pharmacologic agents that can cause iatrogenic harm. There also can be comorbid physical illness, which may be indirectly linked to symptoms from the somatization disorders.
Patients with somatization disorders usually have comorbid psychiatric illness including depressive and anxiety disorders. A general population study3 found that 22% of patients with either somatization or pain disorder had at least 1 comorbid psychiatric diagnosis. Some patients with somatization disorders may present with life-threatening symptoms of severe depression. Life-threatening depression is recognized as a first-line indication for electroconvulsive therapy (ECT), which has proven efficacy in such presentations.4
In the DSM-IV classification system, somatoform disorders included somatization disorder, conversion disorder, undifferentiated somatoform disorder, pain disorder, hypochondriasis, body dysmorphic disorder, and somatoform disorder not otherwise specified.5 However, in the DSM-5, the broad category of somatic symptom and related disorders includes somatic symptom disorder, illness anxiety disorder, conversion disorder, psychological factors affecting other medical conditions, factitious disorder, factitious disorder imposed upon another, other specified somatic symptom and related disorder, and unspecified somatic symptom and related disorder.5
Somatization disorder in the DSM-IV is now recognized as somatic symptom disorder in the DSM-5.2,5 The somatic symptom disorder diagnosis is less stringent with focus on the consequence of dysfunctional thoughts, feelings, and behaviors.2 Clinicians sometimes experience difficulty differentiating between somatization and conversion disorders. There are differences between somatization disorders and conversion disorders. Conversion disorder is typically monosymptomatic and involves the voluntary motor system. In conversion disorder, there are neurologic features, and patients commonly present to general neurology clinics.5 In this article, we will only focus on the efficacy of ECT for somatization disorder. We will amalgamate data from the most recent literature to make recommendations on the use of ECT in patients with refractory somatization disorders.
METHODS
A comprehensive evidence search of the published literature in the last 10 years (2010–2020) was conducted using the search terms ECT, electroconvulsive therapy, efficacy, use of ECT, chronic pain, somatoform disorders, somatoform pain, somatic symptom disorder, and somatization disorder. Our review was limited to articles written in the English language. Databases searched included PsycInfo, MEDLINE, PubMed, Embase, and Google Scholar. A supplementary citation search was also conducted by analyzing the reference lists of identified articles.
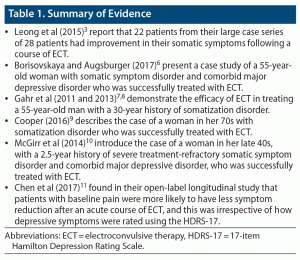
The initial search revealed 31 articles of potential relevance. However, after individually evaluating these articles, we found 5 single case studies, 1 large case series, and 1 open longitudinal study focusing on the use of ECT in somatization disorders.3,6–11 There were no controlled trials or systematic reviews, and the evidence we collated was of low quality.
RESULTS
Case Series: Leong et al, 20153
The authors3 present a large case series of 28 patients who were treated with ECT for somatic symptom and related disorder or somatoform disorders. They reported that 22 of these patients had significant improvement in their somatic symptoms after a course of ECT.3 They conducted a retrospective chart review of all patients treated with an index course of ECT at a university hospital during 2000–2010. They used the broad DSM-IV classification system for somatoform disorder in their study.3
Twenty eight participants including 16 female and 12 male subjects were included in this study. The mean age of the participants was 48.7 years. All patients were unemployed, with the mean length of unemployment calculated as 6.05 years. Fourteen patients had a diagnosis of conversion disorder, 3 had pain disorder, 9 had comorbid pain and conversion disorder, and 2 had a somatoform disorder not otherwise specified. The mean number of psychiatric comorbidities and medical comorbidities was 2.5 and 4.39, respectively. All patients in the study had comorbid major depressive disorder (MDD), and 10 had a diagnosis of posttraumatic stress disorder. There was a mean of 2.89 antidepressant trials and 2.1 antipsychotic trials before the start of ECT.3
Twenty-one participants were initially treated with right unilateral electrode placement, with 14 switching to bilateral electrode placement because of inadequate clinical response. Six participants were initially treated with bifrontal ECT, and 1 individual was treated with bitemporal ECT. Subjects had between 3 and 22 sessions of ECT, and the median number of treatments was 11.5.3
Eighteen of 21 patients reported an improvement in their functional neurologic symptoms; however, 2 reported no change and 1 said that there had been deterioration following ECT. Eleven of 14 participants reported improvement in pain severity, whereas 3 reported worsening of symptoms. One of the 2 patients with gastrointestinal (GI) symptoms showed improvement and the other discontinued ECT early because of no change. Finally, 1 subject described positive improvement in their cardiopulmonary symptoms. Many of the subjects reported improved mood, which was measured using the Geriatric Depression Scale12 and Beck Depression Inventory (BDI)13 rating scales.
There was no control for medication adjustment and psychological interventions during the ECT course. The authors3 stated that the outcome measures used to monitor changes in mood were inconsistent. They add that there may have been potential bias influencing the patients’ subjective improvement in somatic symptoms.3 Overall, the data from this study were of low quality but showed promise for use of ECT.
Case Study: Borisovskaya and Augsburger, 20176
The authors6 discuss the case of a 55-year-old woman with somatic symptom disorder and comorbid MDD who was successfully treated with ECT. There was significant improvement in both her pain and GI symptoms. She had been admitted to a psychiatric ward with a 5-month history of weight loss (roughly 40 lb) with a background of debilitating nausea and abdominal pain. A week before this admission, she had been treated at a different psychiatric unit for suicidal ideation and low mood. She was unable to eat or work as a result of somatic symptoms.6
The patient’s BDI score at the most recent hospital admission was 56, which indicated a severe depressive episode.6 The mental state examination showed signs of psychomotor agitation and depressed mood. With her consent, ECT was started because of the resistant nature of her severe affective disorder and persistent suicidal ideation. She had right unilateral ECT 3 times a week.6
The patient’s mood began to improve after the third ECT treatment. After the fifth session, there was further improvement in her mood with amelioration of her somatic symptoms. She had a total of 7 ECT treatments with remission of her depressive illness (post-ECT BDI score was 7) and suicidality and significant improvement in GI symptoms. At 2-month follow-up, her improvement was maintained with euthymic mood and an absence of GI symptoms.6
Case Study: Gahr et al, 20117 and 20138
The authors7,8 highlight the efficacy of ECT in treating a 55-year-old man with a 30-year history of somatization disorder. He had extensive medical investigations and surgical procedures during the course of his illness. At admission, there were multiple somatic complaints. After 3 months of various treatment trials including both pharmacologic and psychological interventions, he was initiated on a trial of right unilateral ECT.
After 5 sessions, the course was discontinued after he developed severe ECT-related hypertension with systolic blood pressure > 220 mm Hg. However, the patient reported rapid and significant improvement in his somatic symptoms both during and after the ECT course. This improvement was confirmed by his scores on various standardized instruments. His score on the Hamilton Depression Rating Scale (HDRS)14 decreased from 15 to 14. The effect of ECT on mood was negligible, which was consistent with the treating team observation.7,8
The patient’s Whiteley Index score15 decreased (8 to 5), which is a self-rating instrument for hypochondria. His score on the Quantification Inventory for somatoform syndromes, clinical version (QUISS)16 decreased from 54 pre-ECT to 20 following ECT. Wedekind et al16 describe the QUISS as a severity scale for patients fulfilling DSM-IV or ICD-10 diagnostic criteria for somatoform disorders. There was also a reduction from 60 to 26 in his Screening for Somatoform Symptoms-7 (SOMS-7) score. Rief and Hill17 define the SOMS-7 as a 53-item instrument that covers all the somatic symptoms from the DSM-IV and ICD-10. The treatment benefits were sustained at 4-month follow-up.
Case Study: Cooper, 20169
Cooper9 presents the case of a woman in her 70s who was successfully treated with ECT for somatization disorder. She presented with a 4-year history of persisting right upper quadrant abdominal pain and discomfort. There were associated symptoms of depression.9
Nine months before the most recent psychiatric consultation, she had been treated with 6 sessions of bitemporal ECT at another facility. Both the patient and her partner reported that this treatment had been beneficial in terms of providing pain relief and was the only effective treatment to date. She had experienced significant short-term memory impairment during the course of ECT but returned to normal functioning within 1 to 2 weeks. Her somatic symptoms and mood were stable for several months before slowly deteriorating.9
On the mental state examination, there was psychomotor retardation, a flat affect, and poor attention and concentration. She scored 18/30 on the Montreal Cognitive Assessment (MoCA)18 and 19 on the BDI. She had 8 sessions of right unilateral ultrabrief pulse ECT treatments (to minimize cognitive side effects) 3 times/week.9
There was marked improvement in her mood and chronic pain. After the fifth treatment, she noted that the pain had become a sensation that no longer caused her trouble. The discomfort eventually remitted completely. Her BDI score decreased to 5, and she was described as full and bright in affect. She requested maintenance ECT and received this treatment once every third week. The pharmacologic treatments were discontinued, and her BDI score eventually decreased to 0. She was pain free for 7 months, and there was marked improvement in her cognitive performance. Her MoCA score increased to 23 after 6 treatments, and she scored 26 when she had maintenance ECT. There was no subjective impairment in cognitive function, and her partner had no concerns. Her functional ability was at its highest in the last 3 years.9
After 7 months, there was a recurrence of the pain. She declined an increase in the frequency of ECT or to start an acute course. She returned to the ECT clinic after 3 months with a history of worsening and debilitating pain. She fulfilled the criteria for a major depressive episode, although her BDI score was 6. She had an acute course of twice-weekly right unilateral ECT. After 11 treatments, her pain improved and her BDI score decreased to 2. She continued maintenance ECT, which was initially weekly then every 2 weeks. Cooper9 concluded that pain symptoms secondary to depression may improve with ECT.
Case Study: McGirr et al, 201410
The authors describe the case of a woman in her late 40s, with a 2.5-year history of severe treatment-refractory somatic symptom disorder and comorbid MDD, who was successfully treated with ECT. She had symptoms of an idiopathic burning mouth syndrome.10
The glossodynia and stomatodynia caused her intolerable distress and resulted in 14 separate visits to the emergency department and absence from work. On a visual analog scale, she rated the intensity of her pain to be 10/10. Her speech was impaired and her diet was restricted, which resulted in > 10% loss of her body mass index over a 6-month period. She had multiple interventions, which included both medical and psychological treatments.
The patient consented to treatment with ECT. Prior to ECT, her HDRS-29 score was 29 and her MoCA score was 26/30.10 She had 10 sessions of twice-weekly bilateral ECT followed by 10 sessions of continuation ECT with no complication. There was noticeable objective and subjective clinical response by the seventh ECT treatment.
After the index course of ECT, her pain severity score on the visual analogue scale fell to 2/10. Her HDRS-29 score was 6, and her performance on the MoCA improved with a score of 28/30. During the course of continuation ECT, she was euthymic in mood but reported a slight deterioration in pain with a visual analogue scale score of 2 to 3/10. She returned to work and discontinued ECT because of concerns about the effects on work performance.10 There is no information available to establish if she received follow-up and whether her treatment response was sustained.
Open-Label Longitudinal Study: Chen et al, 201711
Chen et al11 explored the predictors associated with ECT outcome in patients with MDD. They found that baseline pain was a significant predictor. They conducted an open-label longitudinal study of 130 psychiatric inpatients with a diagnosis of MDD who were treated with ECT. Baseline clinical characteristics including pain severity were determined. They used standardized rating scales to assess symptom severity. The Body Pain Index (BPI) of the Medical Outcomes Study Short-Form-36 (SF-36),19 particularly items 7 and 8, was used to rate pain severity and pain interference over the month before ECT. The SF-36 measures health status and patient outcomes.20
Bitemporal ECT was generally performed for a maximum of 12 sessions, with 14 patients excluded because they did not complete the first 3 sessions of ECT. The authors found that patients with baseline pain were more likely to have less symptom reduction after an acute course of ECT, and this was irrespective of how depressive symptoms were rated using the HDRS-17. Each point increase on the BPI, which signifies less pain, in the treatment duration, significantly decreased the HDRS-17 score by an average of 0.04 points. The Clinical Global Impressions–Severity of Illness21 score also decreased by 0.01 points with each point increase on the BPI. The authors discuss the limitations of the self-rated pain scale they used, which they felt inadequately measured the patient’s attitude/beliefs and the site of pain.11
CONCLUSIONS
There is case-level evidence supporting the efficacy of ECT in severe and treatment-refractory somatization disorders. The evidence accrued suggests that benefits from ECT are not sustained and maintenance treatment may warrant consideration (Table 1). The case series by Leong et al3 provides the most compelling evidence for the benefits of ECT in this patient group. Overall, the current data are of low quality, and one cannot exclude publication bias. The mechanism of action for ECT in somatoform disorder is unclear. Both major depressive disorder and somatoform disorders may share similar abnormalities in both neuroendocrine and immunologic systems, which may improve with ECT.3
Patients with somatoform disorder commonly have comorbid mental disorders.2,3 ECT is a well-established treatment for affective and psychotic disorders.4 The improvement in symptoms originating from these disorders may improve the overall clinical phenotype. However, the study by Chen et al11 suggests that patients with comorbid depression and somatoform disorders have worse outcomes with ECT than those with isolated depressive disorders.
We conclude that clinicians may consider ECT in severe or life-threatening forms of somatoform disorder that present with comorbid psychiatric disorder. The most recent evidence indicates that ECT may be an effective treatment in somatoform disorders. However, further studies of robust methodology are required before ECT can be considered a treatment option for isolated manifestations of somatoform disorder.
Submitted: August 30, 2020; accepted October 30, 2020.
Published online: May 20, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Acknowledgments: The authors thank Folu Ojutalayo, MSc (clinical librarian, North East London NHS Foundation Trust, Audrey Keep Library and Knowledge Service, Ilford, Goodmayes Hospital site) for his support with the literature search. Mr Ojutalayo reports no conflicts of interest related to the subject of this article.
Clinical Points
- There is case-level evidence that supports the efficacy of electroconvulsive therapy (ECT) in somatization disorders.
- Comorbid somatization disorder in patients with severe depression may be a predictor of poorer response to ECT.
- Clinicians may consider ECT in severe and refractory manifestations of somatization disorder that present with comorbid affective or psychotic disorders.
- There are not enough robust data to suggest ECT as a treatment alternative in isolated somatization disorders.
References (21)

- Kleinstäuber M, Witthöft M, Steffanowski A, et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev. 2014;(11):CD010628. PubMed
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016;2016:bcr2015212553. PubMed CrossRef
- Leong K, Tham JC, Scamvougeras A, et al. Electroconvulsive therapy treatment in patients with somatic symptom and related disorders. Neuropsychiatr Dis Treat. 2015;11:2565–2572. PubMed CrossRef
- Statement on Electroconvulsive Therapy (ECT). Position statement CERT01/17. Royal College of Psychiatrists. February 2017. Accessed April 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/about-us/who-we-are/electroconvulsive-therapy---ect-ctee-statement-feb17.pdf?sfvrsn=2f4a94f9_2
- Stonnington C, Driver-Dunckley E, Noe KH, et al. Conversion and somatic symptom disorders. BMJ Best Practice. 2018. Accessed April 24, 2021. https://bestpractice.bmj.com/topics/en-us/989
- Borisovskaya A, Augsburger JA. Somatic symptom disorder treated with electroconvulsive therapy. Pain Manag. 2017;7(3):167–170. PubMed CrossRef
- Gahr M, Schönfeldt-Lecuona C, Connemann BJ. Somatization disorder treated with electroconvulsive therapy. J ECT. 2011;27(3):266–267. PubMed CrossRef
- Gahr M, Schönfeldt-Lecuona C, Kölle MA, et al. Electroconvulsive therapy in patients with diagnoses other than major depression and/or difficult characteristics: a combined psychiatric-anesthesiological approach based on a retrospective chart analysis. Psychiatry Res. 2013;210(1):159–165. PubMed CrossRef
- Cooper JJ. Recurrent right upper quadrant pain responsive only to electroconvulsive therapy. J ECT. 2016;32(3):e21–e22. PubMed CrossRef
- McGirr A, Davis L, Vila-Rodriguez F. Idiopathic burning mouth syndrome: a common treatment-refractory somatoform condition responsive to ECT. Psychiatry Res. 2014;216(1):158–159. PubMed CrossRef
- Chen CC, Lin CH, Yang WC, et al. Clinical factors related to acute electroconvulsive therapy outcome for patients with major depressive disorder. Int Clin Psychopharmacol. 2017;32(3):127–134. PubMed CrossRef
- Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. PubMed CrossRef
- Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49.
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. PubMed CrossRef
- Conradt M, Cavanagh M, Franklin J, et al. Dimensionality of the Whiteley Index: assessment of hypochondriasis in an Australian sample of primary care patients. J Psychosom Res. 2006;60(2):137–143. PubMed CrossRef
- Wedekind D, Bandelow B, Fentzahn E, et al. The Quantification Inventory for Somatoform Syndromes (QUISS): a novel instrument for the assessment of severity. Eur Arch Psychiatry Clin Neurosci. 2007;257(3):153–163. PubMed CrossRef
- Rief W, Hiller W. A new approach to the assessment of the treatment effects of somatoform disorders. Psychosomatics. 2003;44(6):492–498. PubMed CrossRef
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. PubMed CrossRef
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. PubMed CrossRef
- Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4:2050312116671725. PubMed CrossRef
- Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. DHEW Publication No. 76-338. Rockville, MD: National Institute of Mental Health; 1976:217–222.
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top