ABSTRACT
Objective: To determine if external ear anomalies (EEAs) and minor physical anomalies (MPAs) are more prevalent in patients with depressive disorder than in healthy controls.
Methods: This cross-sectional study was conducted at a tertiary-level referral center between October 1, 2019, and September 30, 2020, and included 100 patients with depressive disorder (diagnosed per ICD–10 criteria) and 100 aged- and sex-matched healthy controls. The study participants were examined using the External Ear Anomalies Assessment Scale and the extended Waldrop Scale.
Results: Independent samples Mann-Whitney U test showed a higher prevalence of mean EEAs and MPAs in patients with depressive disorder. Adherent ear lobe was the most common ear anomaly in both patients (52%) and controls (41%), followed by Darwinian tubercle (21% in the patient group and 19% in the control group).
Conclusions: External ear anomalies are more prevalent in patients with depressive disorder, supporting the neurodevelopmental theory of depression. These EEAs need further description and attention for possible inclusion in scales that assess minor physical anomalies and may be used as an endophenotypic marker for depression in the future.
Prim Care Companion CNS Disord. 2023;25(4):22m03416
Author affiliations are listed at the end of this article.
Depression is one of the most common mental disorders, causing major impairment1 and leading to increased mortality and morbidity.2,3 The external ear develops from the 6 auricular hillocks around the pharyngeal groove by the first and second branchial arch. These hillocks are fused by the end of 8 weeks’ gestation, leading to the characteristic shape of the ear.4,5 The external ear has a complex structure and shape that is species specific and remarkably constant in its basic normal shape.6 Yet, most of the dysmorphology literature pays poor attention to the accurate description of the ear and the definitions of abnormalities. Some of the external ear anomalies (EEAs) are known to be specific for some illnesses and are useful in their diagnosis (eg, ear lobe creases in Beckwith-Wiedemann syndrome, prominent crus of the helix in fetal alcohol syndrome and Saethre–Chotzen syndrome).4
There are various theories of development of depression and understanding of mood disorders, one of which is the “neurodevelopmental theory” that focuses on epigenetic mechanisms reflecting inherited changes in gene expression.7 The neurodevelopmental theory has been studied more in schizophrenia than in depressive disorders.8 There are a few factors that define the neurodevelopmental origin of schizophrenia, termed curious epiphenomena of schizophrenia,9 such as neuromotor anomalies and minor physical anomalies (MPAs), including EEAs.10 The similarities in origin between schizophrenia and major depressive disorder and bipolar disorder have raised questions regarding the role of neurodevelopment.11,12 Among the MPAs, the external ear is one of the most important in studying the major malformations as well as minor anomalies of phenotypic variations.4 It seems possible that a careful examination and detailed description of the ear in those with malformations and/or dysmorphic signs may prove to be of diagnostic value in specific syndromes that may have a neuroectodermal origin in the external ear and the brain.6 A study by Praharaj et al13 found that among the ear abnormalities, prominent crux of helix and abnormal anterior surface were significantly more prevalent in patients with schizophrenia compared to those with bipolar disorder, while the frequency of asymmetrical ears and auricular pits was more common in patients with bipolar disorder. They concluded that prominent crux of helix and ear lobe crease could differentiate patients with schizophrenia and bipolar disorder.13
The MPAs are clinically or cosmetically insignificant errors that occur during the development of morphologic structures such as the mouth, ears, eyes, head, hands, and feet. MPAs develop during the early stage of gestation but persist into adulthood and can easily be detected or observed by careful examination of the defined area.14 As the structures that express MPAs are of the same embryonic origin as the central nervous system, MPAs are valuable biological markers of abnormal brain development.15,16
We conducted a cross-sectional study to explore various EEAs in patients with major depressive disorder compared to a healthy control group. The aim of the study was to evaluate EEAs in patients with depression and their relation to the overall frequency of MPAs and other clinical variables.
METHODS
This cross-sectional study was conducted at a tertiary-level referral center between October 1, 2019, and September 30, 2020. The study was approved by the Institute Ethics Committee of All India Institute of Medical Sciences (AIIMS), Raipur, India (IEC no. AIIMSRPR/IEC/2019/338). Written informed consent was obtained from all participants before registering them for the study.
Participants
Patients were recruited by purposive sampling from the outpatient and inpatient services of AIIMS, Raipur. The sample included 114 patients aged 18–65 years with a diagnosis of depressive disorder (first depressive episode/bipolar/recurrent) in any phase of illness as per ICD-10 diagnostic criteria for research. Patients who were unwilling or had a history of neurologic illness, organic brain disorders, mental retardation, dementia, any substance dependence except for caffeine and tobacco, other psychiatric disorder, disruptive behavior, any physical trauma, or surgery that would affect the EEAs and MPAs were excluded from the study. The final sample included 100 patients. The healthy control group included 100 age- and gender-matched participants recruited from the hospital staff and general medicine outpatient department (attenders with patients) who were not first-degree relatives of the patients.
Clinical Assessment
All the required sociodemographic data were collected from the participants after written consent was obtained. Severity of depressive episode was assessed by the Hamilton Depression Rating Scale (HDRS).17 Healthy controls were screened with the General Health Questionnaire-518,19; only those who scored < 2 were included in the study. To assess the EEAs, the External Ear Anomalies Assessment Scale developed by Praharaj et al13 was used. A modified version of the Waldrop Scale, the extended Waldrop Scale,20,21 was used to assess the minor physical anomalies in all the participants. All 54 items of the extended Waldrop Scale were scored as absent or present, and all the participants were assessed for MPAs by a trained rater (P.K.S.).
Statistical Analysis
The Shapiro-Wilk test was used to check whether continuous variables were in a normal distribution. For both groups, differences for categorical and continuous variables were computed using the χ2 test and independent samples Mann-Whitney U test, respectively. Comparison among types of depression (first episode/bipolar/recurrent) was done using the Kruskal-Wallis test (1-way analysis of variance). Spearman correlation coefficients were computed between MPA scores and External Ear Anomalies Assessment Scale scores. Statistical analysis was done using SPSS 23.0. The level of significance was kept at .05.
RESULTS
The mean ± SD age of the patient group was 34.4 ± 10.9 years and of the control group was 32.07 ± 11.2 years (Mann-Whitney U = 0.60). Most of the patient population were male (69%) and Hindu (93%). There was a significant difference in occupational and family income status of patients and controls (P < .00), with more of the controls being employed and having family incomes > 10,000 INR (Indian rupees)/month. For the patient group, the mean ± SD duration of illness was 2.55 ± 3.29 years, with an age at onset of 31.79 ± 10.70 years. Most of the patients had first-episode depression (64%), while 8% had bipolar disorder and 28% had recurrent depressive disorder.
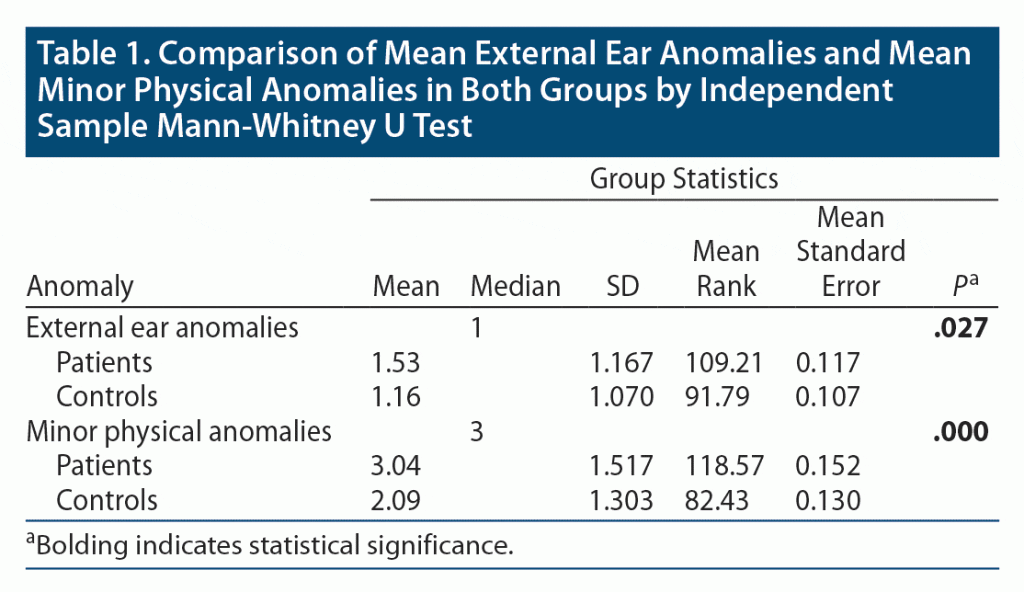
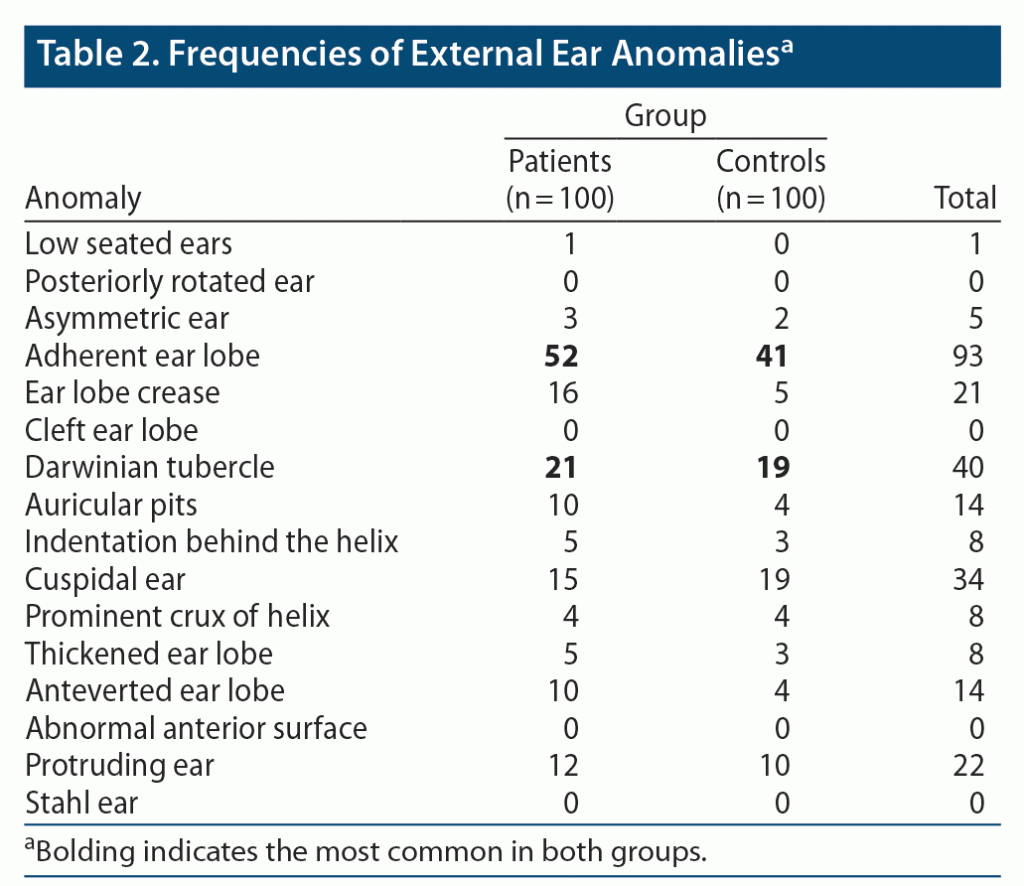
When comparing ear anomalies in the 2 groups, no significant difference in the frequencies of total number of ear anomalies was seen between patients and controls. Regarding mean EEAs, the patients had significantly higher mean anomalies than controls (patients: mean EEAs = 1.53; controls: mean EEAs = 1.16; P < .05) (Table 1). Among the EEAs, adherent ear lobe was the most common ear anomaly in both patients (52%) and controls (41%), followed by Darwinian tubercle (21% in the case group and 19% in the control group) (Table 2).
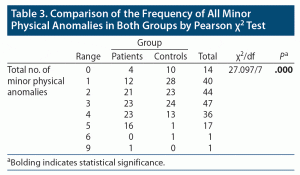
Comparison of the frequencies of minor physical anomalies revealed a significantly (P < .00) higher number of total MPAs (≥ 3 total MPAs) in patients with depressive disorder than in the control group (Table 3). There were significant differences (P < .05) in mean MPAs between patients (mean MPAs = 3.04) and controls (mean MPAs = 2.09) (Table 1). Among the total MPAs, adherent ear lobes were the most frequent anomaly (52% in cases and 41% in controls), followed by wide distance between the first and second toe (41% in cases and 9% in controls) and confluent eyebrows (29% in cases and 18% in controls).
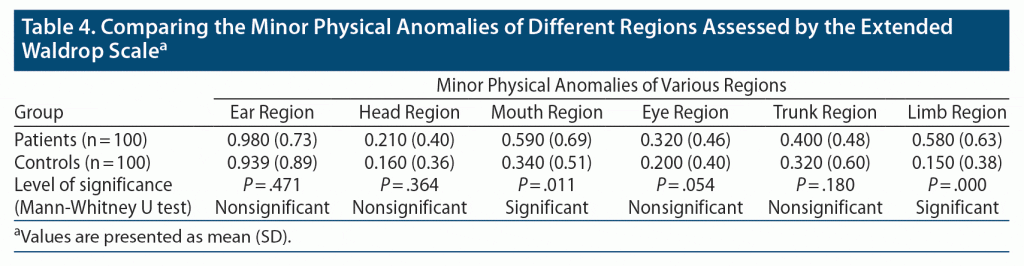
Among different regions of the body per the extended Waldrop Scale, a significant difference (P < .05) in MPAs of the mouth (mean mouth anomalies: 0.59 in the case group and 0.34 in the control group) and limb (mean limb anomalies: 0.58 in the case group and 0.15 in the control group) regions were seen in both groups (Table 4). Higher number of total MPAs was significantly associated with higher number of total EEAs (Spearman correlation coefficient: 0.320) (P < .00).
Regarding gender in the patient group, there was a significant difference (P = .037) in HDRS scores (18.85 in males and 21.78 in females) and mean MPAs (2.72 in males and 2.23 in females). When both groups were divided on their median basis, there was a significant difference (P = .00) for total MPAs, as 40% of the patient group were above median, while only 15% of the control group had total MPAs above the median value.
DISCUSSION
This pilot study revealed that among external ear anomalies, adherent earlobe, Darwinian tubercle, earlobe crease, and anteverted ear lobe were more prevalent in the patient group than in the control group, although most of these anomalies were not included in the standard MPA scales. The groups had significantly different numbers of total mean EEAs and MPAs (patients = 1.53, controls = 1.16, P < .027) and mean total MPAs (patients = 3.04, controls = 2.09, P < .000).
Per these results, we can hypothesize that EEAs and MPAs are found predominantly in patients with depressive disorders compared to healthy controls. Based on earlier studies and the literature, these findings suggest a similar origin of brain and ear anomalies that point toward the neurodevelopmental origin of depression.8,9 EEAs can be assessed as an endophenotype for depressive disorder due to their higher prevalence in patients with depression. By assessing EEAs and MPAs, we may be able to identify populations who are prone to develop depression; however, this does not aid in diagnosis, as it is a nonspecific indicator for depressive disorder. The clinical relevance of EEAs and MPAs in the differential diagnosis and as a prognostic indicator is not yet clear. They can be used as an endophenotypic marker for depression or as a screening tool in high-risk populations for which further evaluation is to be done.
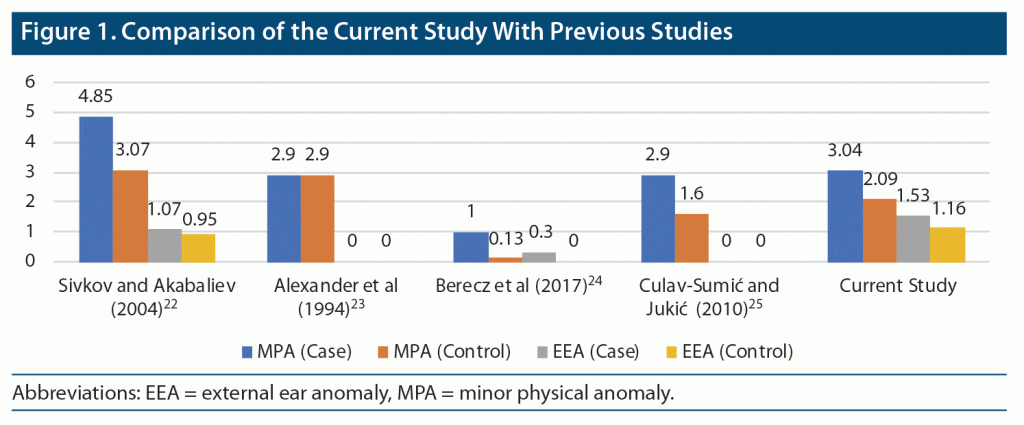
Figure 1 provides a comparison of the current and previous studies. The results of Sivkov and Akabaliev22 and Culav-Sumić and Jukić25 regarding mean total MPAs are comparable to our study; however, we found no previous studies correlating the EEAs in patients with depressive disorders.
In our study, patients with depressive disorder showed an upward shift in the frequency distribution of total MPAs, which denoted a high anomaly load in this group. Correlation of the frequencies of total number of MPAs and of EEAs by Spearman correlation coefficient showed that a higher number of total MPAs were significantly (P < .000) associated with a higher number of EEAs. Hence, assessment of ear anomalies can be an easier and more efficient way to assess neurodevelopmental defects.
Our study revealed a significant difference in mean MPAs of the mouth (P < .011) and limb regions (P < .000) between patients with depressive disorders and the control group. However, we could not establish a significant difference in mean ear anomalies as per standard scales of MPAs (extended Waldrop Scale), but we could significantly differentiate the mean EEAs in both groups using the External Ear Anomalies Assessment Scale. This finding indicates that current standards are still restricted with regard to range and lack the accuracy to assess anomalies related to the ear region. These EEAs are worth including in scales that assess minor physical anomalies.
Strength and Limitations
A strength of this study is the larger sample size. We also included definitions of ear anomalies (Appendix 1) along with images of anomalies found in the study groups (Appendix 2). However, our study has some limitations, as we defined the MPAs as either “present” or “absent,” while some studies such as Akabaliev et al26 used a graded manner of assessment for some of the MPAs like fine electric hair, head circumference, epicanthus, intercanthal distance, low-seated ears, and high palate.24 Our evaluation for EEAs was not blinded and was done by only 1 investigator. A majority of the patient population had first-episode depression for which further follow-up could not be done, as some of those patients would develop a manic/hypomanic episode that would change the diagnosis to bipolar disorder. Other limitations of the study include the sample size not meeting the criteria for an effective sample size and that the evaluation was conducted by only one investigator. A design in which 2 or more raters independently evaluated subjects using a blinding protocol would be a great improvement.
CONCLUSION
External ear anomalies are more prevalent in patients with depressive disorder, supporting the neurodevelopmental theory of depression. These EEAs need further description and attention for possible inclusion in scales that assess minor physical anomalies, which will strengthen the literature and their use as an endophenotypic marker for depression in the future.
Article Information
Published Online: July 20, 2023. https://doi.org/10.4088/PCC.22m03416
© 2023 Physicians Postgraduate Press, Inc.
Submitted: September 12, 2022; accepted January 31, 2023.
To Cite: Soni PK, Singh LK, Arora RD, et al. External ear anomalies and minor physical anomalies in depressive disorder patients and healthy controls. Prim Care Companion CNS Disord. 2023;25(4):22m03416.
Author Affiliations: All India Institute of Medical Sciences, Tatabandh, Raipur, India (Soni, Singh, Arora, Tikka, Mandal, Nandan, Das).
Corresponding Author: Lokesh Kumar Singh, MD, DPM, All India Institute of Medical Sciences, Tatabandh, Raipur 492099 India ([email protected]).
Funding/Support: None.
Supplementary Material: See accompanying pages.
CLINICAL POINTS:
- This pilot study showed that mean external ear anomalies (EEAs) and minor physical anomalies (MPAs) are more prevalent in patients with depressive disorder in comparison to healthy controls.
- EEAs can be used as an endophenotypic marker for future prospective studies and should be a focus in the development of scales assessing MPAs, which are currently lacking in this area.
References (26)

- Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients. results from the Medical Outcomes Study. JAMA. 1989;262(7):914–919. PubMed CrossRef
- Choo C, Diederich J, Song I, et al. Cluster analysis reveals risk factors for repeated suicide attempts in a multi-ethnic Asian population. Asian J Psychiatr. 2014;8(1):38–42. PubMed CrossRef
- Large M. Study on suicide risk assessment in mental illness underestimates inpatient suicide risk. BMJ. 2016;532:i267. PubMed CrossRef
- Stevenson RE, Hall JG, eds. Human Malformations and Related Anomalies. Oxford University Press; 2005.
- Kumar P, Burton BK. Congenital Malformations Evidence-Based Evaluation and Management. McGraw-Hill; 2008.
- Hunter AG, Yotsuyanagi T. The external ear: more attention to detail may aid syndrome diagnosis and contribute answers to embryological questions. Am J Med Genet A. 2005;135A(3):237–250. PubMed CrossRef
- Fuemmeler BF, Lee CT, Soubry A, et al. DNA methylation of regulatory regions of imprinted genes at birth and its relation to infant temperament. Genet Epigenet. 2016;8:59–67. PubMed CrossRef
- Owen MJ, O’Donovan MC, Thapar A, et al. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198(3):173–175. PubMed CrossRef
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed). 1987;295(6600):681–682. PubMed CrossRef
- Jablensky A, McNeil TF, Morgan VA. Barbara Fish and a short history of the neurodevelopmental hypothesis of schizophrenia. Schizophr Bull. 2017;43(6):1158–1163. PubMed CrossRef
- Palomo T, Kostrzewa RM, Archer T, et al. Neurodevelopmental liabilities in schizophrenia and affective disorders. Neurotox Res. 2002;4(5-6):397–408. PubMed CrossRef
- Brown N. Clinical Governance in Mental Health and Learning Disability Services: A Practical Guide. James A, Worrall A, Kendall T, eds. London: Gaskell. British Journal of Psychiatry. 2006;189(1):88.
- Praharaj SK, Sarkar S, Sinha VK. External ear abnormalities in existing scales for minor physical anomalies: are they enough? Psychiatry Res. 2012;198(2):324–326. PubMed CrossRef
- Ismail B, Cantor-Graae E, McNeil TF. Minor physical anomalies in schizophrenic patients and their siblings. Am J Psychiatry. 1998;155(12):1695–1702. PubMed CrossRef
- Cotter D, Pariante CM. Stress and the progression of the developmental hypothesis of schizophrenia. Br J Psychiatry. 2002;181(5):363–365. PubMed CrossRef
- Pantelis C, Yücel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31(3):672–696. Published online Jul 14, 2005. PubMed CrossRef
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. PubMed CrossRef
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire; a Technique for the Identification and Assessment of Non-Psychotic Psychiatric Illness. London: Oxford University Press; 1972.
- Shamasunder C, Sriram TG, Murali Raj SG, et al. Validity of a short 5-item version of the general health questionnaire (g.h.q). Indian J Psychiatry. 1986;28(3):217–219. PubMed
- Mehes K. Informative Morphogenetic Variants in the Newborn. Budapest: Akademiai Kiado; 1998.
- Trixler M, Tenyi T, Csabi G, et al. Minor physical anomalies in schizophrenia and bipolar affective disorder. Schizophr Res. 2001;52(3):195–201. PubMed CrossRef
- Sivkov ST, Akabaliev VH. Discriminating value of total minor physical anomaly score on the Waldrop physical anomaly scale between schizophrenia patients and normal control subjects. Schizophr Bull. 2004;30(2):361–366. PubMed CrossRef
- Alexander RC, Mukherjee S, Richter J, et al. Minor physical anomalies in schizophrenia. J Nerv Ment Dis. 1994;182(11):639–644. PubMed CrossRef
- Berecz H, Csábi G, Herold R, et al. Minor physical anomalies and dermatoglyphic signs in affective disorders: a systematic review. Psychiatr Hung. 2017;32(1):108–127. PubMed
- Culav-Sumić J, Jukić V. Minor physical anomalies in women with recurrent unipolar depression. Psychiatry Res. 2010;176(1):22–25. PubMed CrossRef
- Akabaliev V, Sivkov S, Mantarkov M, et al. Minor physical anomalies in patients with bipolar I disorder and normal controls. J Affect Disord. 2011;135(1-3):193–200. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top