Objective: To assess (1) the variance of plasma clozapine levels; (2) the relative importance of sex, smoking habits, weight, age, and specific genetic variants of cytochrome P450 1A2 (CYP1A2), uridine diphosphate glucuronosyltransferase 1A4 (UGT1A4), and multidrug resistance protein 1 (MDR1) on plasma levels of clozapine; and (3) the relation between plasma clozapine levels, fasting glucose levels, and waist circumference.
Method: There were 113 patients on clozapine treatment recruited from psychosis outpatient clinics in Stockholm County, Sweden. Patients had genotype testing for single nucleotide polymorphisms: 2 in MDR1, 3 in CYP1A2, and 1 in UGT1A4. Multiple and logistic regression were used to analyze the relations.
Results: There was a wide variation in plasma concentrations of clozapine (mean = 1,615 nmol/L, SD = 1,354 nmol/L), with 37% of the samples within therapeutic range (1,100-2,100 nmol/L). Smokers had significantly lower plasma clozapine concentrations than nonsmokers (P ≤ .03). There was a significant association between the rs762551 A allele of CYP1A2 and lower plasma clozapine concentration (P ≤ .05). Increased fasting glucose level was 3.7-fold more frequent in CC and CA genotypes than AA genotype (odds ratio = 0.27; 95% confidence interval, 0.10-0.72). There was no significant relation between higher fasting glucose levels, larger waist circumference, and higher clozapine levels.
Conclusions: It is difficult to predict plasma clozapine concentration, even when known individual and genetic factors are considered. Therefore, therapeutic drug monitoring is recommended in patients who are treated with clozapine.
Objective: To assess (1) the variance of plasma clozapine levels; (2) the relative importance of sex, smoking habits, weight, age, and specific genetic variants of cytochrome P450 1A2 (CYP1A2), uridine diphosphate glucuronosyltransferase 1A4 (UGT1A4), and multidrug resistance protein 1 (MDR1) on plasma levels of clozapine; and (3) the relation between plasma clozapine levels, fasting glucose levels, and waist circumference.
Method: There were 113 patients on clozapine treatment recruited from psychosis outpatient clinics in Stockholm County, Sweden. Patients had genotype testing for single nucleotide polymorphisms: 2 in MDR1, 3 in CYP1A2, and 1 in UGT1A4. Multiple and logistic regression were used to analyze the relations.
Results: There was a wide variation in plasma concentrations of clozapine (mean = 1,615 nmol/L, SD = 1,354 nmol/L), with 37% of the samples within therapeutic range (1,100-2,100 nmol/L). Smokers had significantly lower plasma clozapine concentrations than nonsmokers (P ≤ .03). There was a significant association between the rs762551 A allele of CYP1A2 and lower plasma clozapine concentration (P ≤ .05). Increased fasting glucose level was 3.7-fold more frequent in CC and CA genotypes than AA genotype (odds ratio = 0.27; 95% confidence interval, 0.10-0.72). There was no significant relation between higher fasting glucose levels, larger waist circumference, and higher clozapine levels.
Conclusions: It is difficult to predict plasma clozapine concentration, even when known individual and genetic factors are considered. Therefore, therapeutic drug monitoring is recommended in patients who are treated with clozapine.
Prim Care Companion CNS Disord 2015;17(1):doi:10.4088/PCC.14m01704
© Copyright 2015 Physicians Postgraduate Press, Inc.
Submitted: July 23, 2014; accepted October 1, 2014.
Published online: February 19, 2015.
Corresponding author: Eric Olsson, MD, Department of Adult Psychiatry, PRIMA Barn och Vuxenpsykiatri AB, Danderyd University Hospital, House 38, Level 1, SE-182 87 Danderyd, Sweden ([email protected]).
Clozapine is an antipsychotic drug that is clinically effective and has limited extrapyramidal adverse events.1,2 Clozapine is effective in patients who have schizophrenia that is resistant to other treatment.3 However, there are adverse events that are more frequent with clozapine than other neuroleptics including weight gain, increased fasting glucose, electroencephalographic changes, and seizures.2 In contrast with other second-generation antipsychotic drugs, clozapine has a dose-response relation between plasma concentration and risk of these adverse events.4-6
Clozapine has wide intraindividual and interindividual variation in plasma concentration with a given dose. Many factors may affect clozapine plasma levels including genetic variants of drug-metabolizing enzymes and transporting proteins, smoking habits, sex, age, concurrent use of other drugs, and food.7-9 It is unknown whether plasma concentration may be predicted from these factors.
The objectives of this study were to assess (1) the variance of plasma clozapine levels; (2) the relative importance of sex, smoking status, weight, age, and specific genetic variants of cytochrome P450 1A2 (CYP1A2), uridine diphosphate glucuronosyltransferase 1A4 (UGT1A4), and multidrug resistance protein 1 (MDR1) on plasma levels of clozapine; and (3) the relation between plasma clozapine level, fasting glucose level, and waist circumference.
METHOD
Study Subjects
All patients who had regular clinical treatment in specialized psychosis clinics were asked to participate in the Swedish Study of Metabolic Risks in Psychosis, performed mainly in Stockholm County, Sweden. These clinics provided treatment for all outpatients in the clinic catchment area who had long-term psychotic disorders. Patients for the current study were recruited to the Swedish Study of Metabolic Risks in Psychosis from 2005 to 2010. Clinical diagnoses were confirmed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).10 Of 731 patients, 113 patients were prescribed clozapine (15%) including 74 patients who were prescribed clozapine as monotherapy. Genetic analysis was performed in 95 of the 113 patients (84%) who took clozapine. Information about dosage of antipsychotic drugs, time between dose and venous blood sampling, concomitant medications, waist circumference, fasting plasma glucose level, body weight, sex, and smoking habits was included in the study database. Daily clozapine dose was recorded for each patient, and concurrent drugs known to alter the activity of CYP1A2 or CYP2D6 were identified to assess potential effects on clozapine blood level. Patients were given written instructions to fast overnight and not to take their prescribed morning dose of clozapine before venous blood sampling. All participants gave informed consent to participate. Ethical approval was given by the Stockholm Regional Ethical Review Committee.

- Routine therapeutic drug monitoring is advised when clozapine is used.
- It is difficult to predict plasma clozapine concentration, even when known factors are considered.
- The risk of increased fasting glucose may be dependent on plasma clozapine concentration.
Plasma Clozapine Concentration
Plasma concentrations of clozapine and its major metabolite norclozapine were measured in 98 patients (87%) with reversed phase high-performance liquid chromatography (HPLC) coupled with ultraviolet detection. Plasma samples (500 µL) were prepared by a 2-step liquid-liquid extraction, starting with extraction to an organic phase followed by back extraction to an acidic water phase, from which an aliquot was injected into the HPLC system. Quantification range was 0.1 to 10 µM for both analytes, and total coefficient of variation was 4.1%-8.3%. Plasma concentrations of clozapine were corrected for daily dose (ratio of concentration to dose [C/D]) based on the linear relation between given dose and achieved plasma concentration as previously reported.10 Therapeutic plasma interval for clozapine was 1,100 to 2,100 nmol/L (360-690 ng/mL; conversion: [ng/mL] = [nmol/L] ׷ 3.06); this was the interval used clinically by the Division of Clinical Pharmacology at the Karolinska University Hospital, Stockholm, Sweden.11-17
Genotyping
The DNA was extracted from venous blood samples (5 mL per sample) using a conventional sodium dodecyl sulfate-urea buffer and quantified with spectrophotometry. Genotyping of the UGT1A4 L48 V allele, with the Valine-encoding G allele expected to cause lower enzyme activity, was performed according to previously published polymerase chain reaction-restriction fragment length polymorphism analyses.18 The primer sequences, rs number, fragment length, and reaction conditions were previously described (Supplementary Table 1).18 Genotyping of the CYP1A2 *1F (increased enzyme activity), *1D (unknown effect on enzyme activity), *1K (decreased enzyme activity), and MDR1 −3435C > T alleles (associated with altered expression of the transport protein) was performed using single-nucleotide polymorphism genotype assays (TaqMan, Life Technologies, Carlsbad, California) (7900HT instrument, Applied Biosystems, Foster City, California) as described previously with specific sequences of interest, rs numbers, and reaction conditions (Supplementary Table 2).19 Genotype determination of the MDR1 −2677G > T allele (associated with altered expression of the transport protein) was performed using pyrosequencing with specific rs numbers, primers (forward, reverse, and sequencing primers), and reaction conditions (Supplementary Table 3).20,21
Statistical Methods
All variables were summarized with descriptive statistics (mean, standard deviation, and frequency). Differences in mean plasma C/D ratio for clozapine, unadjusted and adjusted for differences in time to most recent dose taken, were analyzed with Mann-Whitney test for differences between patients with different sex (men, women), age (< 44 y, ≥ 44 y), smoking status (current smoker, nonsmoker), body weight (< 90 kg, ≥ 90 kg), and genetic variants. The genotypes of the different single-nucleotide polymorphisms were coded under a dominant or recessive model as dichotomous variables.
Multiple regression analysis (stepwise forward) was used to study the combined effect of sex (men, women), smoking status (currently smoking, not smoking), age (< 44 y, ≥ 44 y), weight (continuous), and the different dichotomized genotypes on C/D ratio for clozapine or norclozapine as dependent variable. There were 3 additional regression models created: (1) a logistic regression model (stepwise forward) was created to study the combined effect of genotypes with an association to C/D ratio for clozapine, sex, age, and smoking status on increased fasting plasma glucose level; (2) The same independent variables were entered into a logistic regression model (stepwise forward) with increased waist circumference as dependent variable; the dependent variables “increased fasting plasma glucose” and “increased waist circumference” were dichotomized (increased plasma glucose level defined as > 5.6 mmol/L or use of antidiabetic treatment, and increased waist circumference defined as > 102 cm for men and > 88 cm for women) based on the global definition of the metabolic syndrome22; and (3) A logistic regression model (stepwise forward) also was used to investigate the relation between plasma clozapine levels, fasting glucose levels, and waist circumference. Statistical significance was defined by P ≤ .05.
RESULTS
Daily Dose of Clozapine and Interacting Drugs
Information about daily clozapine dose was available for all 113 patients (Table 1). In 93 patients who provided information about drugs taken concurrently with clozapine, 7 patients were taking medication possibly affecting CYP1A2 or CYP2D6 activity (Table 1). No patients took drugs that may have inhibited CYP1A2 such as fluvoxamine (Table 1).
Plasma Clozapine and Norclozapine Concentration
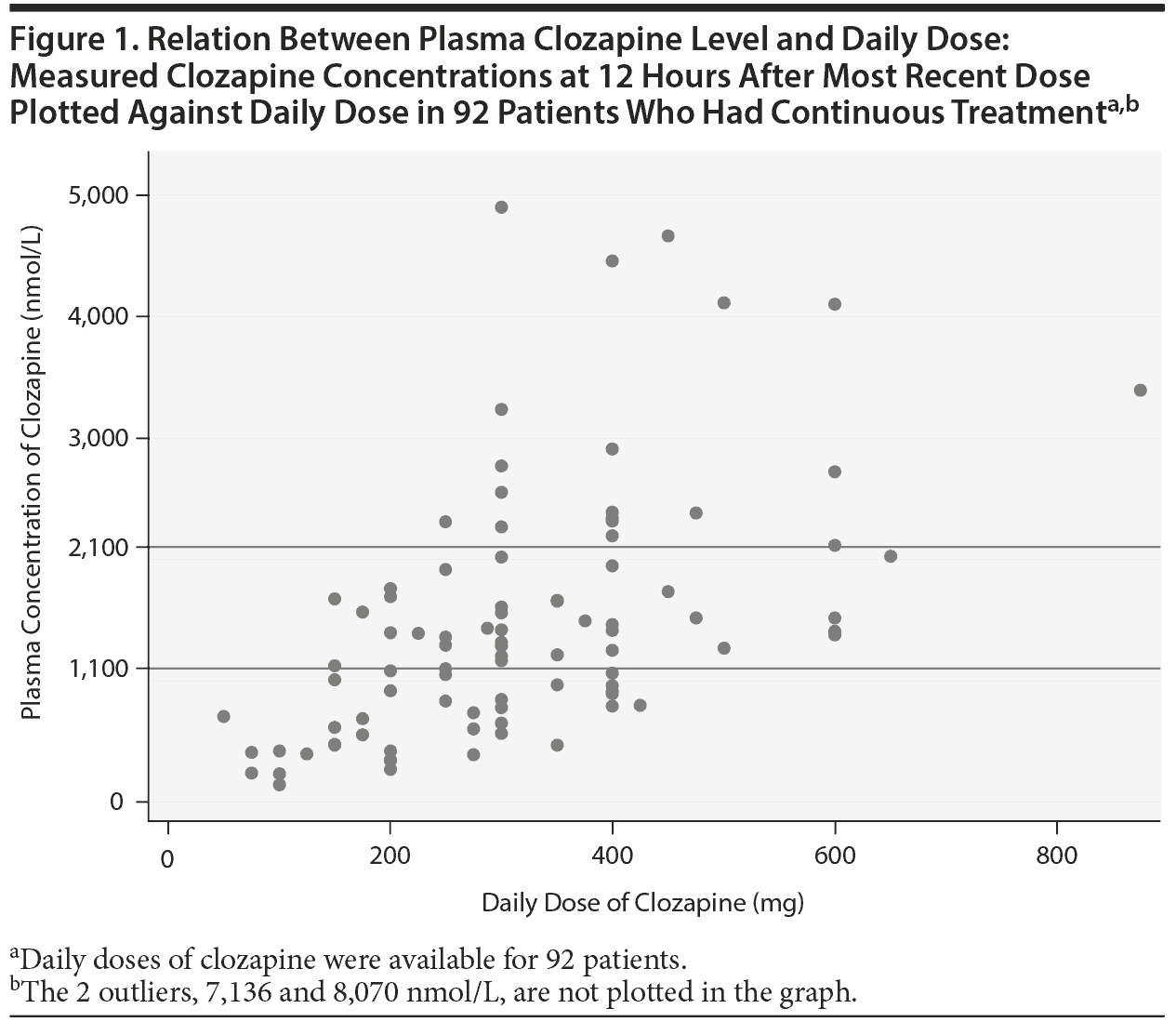
There was a wide variation in clozapine and norclozapine plasma levels (Table 1). There were only 34 patients (37%) who had plasma clozapine levels within the suggested therapeutic range (1,100-2,100 nmol/L), and 22 patients (24%) had plasma levels below the suggested lower limit (< 750 nmol/L) at 12 hours after the most recent dose taken (Figure 1).23 After exclusion of patients who took ≥ 2 antipsychotic drugs, there were 20 patients (22%) who had a clozapine level < 750 nmol/L. There were 9 patients (10%) who had a plasma clozapine level > 3,000 nmol/L and may have required dose reduction, including 2 outliers in the sample who had exceptionally high plasma clozapine levels (8,070 and 7,136 nmol/L).8
Genotype, Patient Characteristics, and Clozapine Concentration
Genotyping was performed on samples from 95 patients. Among the minor alleles of single-nucleotide polymorphisms in CYP1A2 studied, the most common alleles were the C allele of rs762551 (44 patients, allele frequency 28%) and the deletion allele of rs35694136 (9 patients, allele frequency 6%). The T allele of rs12720461 was not detected in any patient. In MDR1, the most common allele was the C allele of rs1045642 (63 patients, allele frequency 41%). In UGT1A4, the Val allele of rs2011425 was present in 11 patients (allele frequency 6%). Genotype frequencies of CYP1A2, UGT1A4, and MDR1 with corresponding mean C/D ratios of clozapine were varied (Supplementary Table 4).
There was a significant difference in mean plasma concentration of clozapine (C/D) between the genotypes of rs762551 in CYP1A2, with lower values for AA genotype (4.2) than AC and CC (6.3; P ≤ .05). The genotypes of the other single nucleotide polymorphisms showed no significant difference in mean C/D (unadjusted for covariates). We found no significant association between mean C/D for clozapine and smoking status, sex, age, or weight (unadjusted for covariates).
Factors Affecting Plasma Clozapine Concentration
There was a significant association between the AA genotype in rs762551 (CYP1A2) and lower plasma clozapine concentration (Table 2). There was a significant association between the GG genotype in rs2032582 (MDR1) and lower plasma clozapine concentration (Table 2). Smoking was associated with lower plasma clozapine concentration (Table 2). The variance of clozapine concentration in the sample was explained by the factors in the regression model only to a limited extent (R2 linear = 0.162).
Factors Affecting Plasma Glucose and Waist Circumference
Odds ratios from the logistic regression (stepwise forward) were used to study the combined effect of genotype in rs762551, rs2032582, and the potential independent variables sex, age, and smoking status on the risk of increased fasting glucose level and increased waist circumference. Increased fasting glucose level was less common among AA genotype in rs762551, but rs2032582 showed no significant association. Increased fasting glucose level was more common in men than women and in older than younger patients (Table 3). Smoking did not affect fasting glucose level in the model, and there was no effect of the different genotypes, sex, age, or smoking status on waist circumference.
DISCUSSION
The present results showed wide variation in plasma clozapine concentration, and few patients had clozapine levels within the therapeutic range (Figure 1). Many patients had plasma clozapine concentrations below the therapeutic range. With specific dose such as 300 mg/d, plasma concentrations may be below, within, or above therapeutic range. The variation in drug levels could be explained only to a limited extent (16%) by genetic variants assessed in this study and smoking habits.
A strength of this study was the inclusion of a population-based clinical sample of patients who were receiving continuous treatment with clozapine. The sample was representative of patients who typically are prescribed clozapine because most patients had schizophrenia (82%), and the distribution of sex and smoking status in the sample was consistent with patient groups in other studies.24 Limitations of the study may include bias because 32% of patients used clozapine in combination with another antipsychotic drug. Another potential limitation of the study was the absence of data about the effect of clozapine treatment. Previous studies showed that intraindividual variability of plasma clozapine concentration may be 32% for heavy smokers and 19% for nonheavy smokers.25 The patients in the present study were informed of blood sampling to study plasma concentrations of clozapine, and this might have affected compliance with drug intake before sampling.
Several factors may affect plasma levels of clozapine. The present results showed that the A allele of the rs762551 polymorphism in CYP1A2 was associated with increased metabolism and lower plasma concentrations of clozapine. In patients who were homozygous for the rs762551 A allele, the strength of the relation was similar to that of smoking status, which may markedly reduce plasma clozapine levels. Patients homozygous for the rs762551 A allele of CYP1A2 also had a lower risk of developing increased fasting glucose level, but this may be a chance finding because of multiple statistical analyses. Among the genetic factors, the strongest association was observed between the AA genotype in rs762551 and low plasma clozapine concentration, consistent with previous findings that suggested a major function of CYP1A2 in the metabolism of clozapine.26
Smoking, a potent inducer of CYP1A2 activity,7,10,27 was associated with lower clozapine C/D ratios in the present study. The induction of CYP1A2 activity is caused by the polycyclic aromatic hydrocarbons in cigarette smoke and not by nicotine.28 Female sex previously had been associated with markedly (40%) increased clozapine concentrations.29,30 In contrast, the present study showed no significant association between sex and clozapine C/D ratios, consistent with another study.31 Studies about CYP1A2 activity usually have shown lower activity in women than men, and CYP1A2 activity is decreased by oral contraceptives. The sex difference in CYP1A2 activity may be specific to ethnicity, and people of European ancestry have shown no sex difference, in contrast with Asians or Africans.32 Some studies have shown increased plasma clozapine levels with increased age,7,30,33 but this was not observed in the present and other previous studies.31,34 These differences may be attributed to shorter age ranges between different studies.
The low proportion of patients (37%) who had plasma clozapine concentration within therapeutic range suggests that treatment was not optimized for adverse events or antipsychotic effects. This result is consistent with results of a recent study of clozapine blood levels in 778 subjects that showed only one-third of patients within the target range and another one-third of patients above or below the target range.35 Therapeutic drug monitoring of clozapine has been recommended internationally but is not performed routinely in Sweden.
Both outliers who had high plasma clozapine concentration were males who were treated with moderate to high daily doses of clozapine (300 mg and 500 mg), were nonsmokers, had no interacting drugs, and carried genotypes in rs762551 and rs2032582 associated with high plasma concentrations of clozapine. Furthermore, both patients had increased fasting glucose, and 1 patient had increased waist circumference.
According to the Swedish Prescribed Drug Register of the National Board of Health and Welfare,36 5,777 patients were prescribed clozapine during 2012, and 551 patients were prescribed clozapine for the first time. Future studies are warranted to investigate whether genotype evaluation may affect clinical outcomes such as time to efficacy and adverse events.
The AA variant of rs762551 in CYP1A2 may be associated with nonresponders to clozapine.37 Many patients in the current sample (54%) were homozygous for the rs762551 A allele. In this study, the AA genotype was associated with lower plasma clozapine concentrations and normal plasma glucose levels (≤ 5.6 mmol/L). Further studies may compare genotypes in CYP1A2 and metabolic measurements in responders and nonresponders to clozapine. There is an association between metabolic changes and clinical effect for olanzapine and risperidone,38 but it is unknown whether this is true for clozapine.
The difficulty to predict plasma clozapine concentration, even when known interacting factors and genetic variants are considered, suggests that therapeutic drug monitoring should be implemented whenever clozapine is used. Individual dosage guided by plasma concentration, antipsychotic effect, and adverse events may optimize treatment and may minimize risk of increased fasting glucose, weight gain, and seizures.
Drug names: carbamazepine (Carbatrol, Equetro, and others), clozapine (Clozaril, FazaClo, and others), fluvoxamine (Luvox and others), metoprolol (Toprol, Lopressor, and others), olanzapine (Zyprexa), omeprazole (Prilosec and others), paroxetine (Paxil, Pexeva, and others), propranolol (Inderal, InnoPran, and others), risperidone (Risperdal and others).
Author affiliations: Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, and Department of Adult Psychiatry, PRIMA Barn och Vuxenpsykiatri AB, Stockholm (Dr Olsson); Department of Psychiatry, Tiohundra AB, Norrtפlje (Drs Edman and זsby and Ms Hukic); Department of Neurobiology, Care Sciences and Society, Centre of Family Medicine, Karolinska Institutet, Stockholm (Drs Edman and זsby); Center for Molecular Medicine, Karolinska University Hospital, Stockholm (Drs Edman, Lavebratt, and זsby); Department of Laboratory Medicine, Division of Clinical Pharmacology, Karolinska Institutet, Stockholm (Dr Bertilsson); Neurogenetics Unit, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm (Drs Hukic and Lavebratt); and Department of Cardiology, Danderyd University Hospital, Karolinska Institutet, Stockholm (Dr Eriksson), Sweden.
Potential conflicts of interest: Dr Olsson has received grant support from PRIMA Child and Adult Psychiatry Inc. Dr ×–sby has received funding for attending courses from Janssen-Cilag and has research collaboration with Lundbeck. Drs Edman, Bertilsson, Lavebratt, and Eriksson and Ms Hukic report no conflicts of interest related to the subject of this article.
Funding/support: This study was supported by a grant from PRIMA Child and Adult Psychiatry Inc. The Swedish Study of Metabolic Risks in Psychosis was supported by ALF grants 20060100 and 20080022 from Stockholm County Council and Karolinska Institutet and grants from the Department of Drug Management and Informatics, Stockholm County Council, and Söderström-Königska Hospital.
Role of the sponsors: No sponsor had any role in design and conduct of the study; collection, treatment, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Acknowledgment: The authors thank research assistant Carina Schmidt for qualified administration of patient samples.
Supplementary material: See accompanying pages.
REFERENCES
1. Vera I, Rezende L, Molina V, et al. Clozapine as treatment of first choice in first psychotic episodes: what do we know? Actas Esp Psiquiatr. 2012;40(5):281-289. PubMed
2. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. PubMed doi:10.1016/S0140-6736(13)60733-3
3. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796. PubMed doi:10.1001/archpsyc.1988.01800330013001
4. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? a literature review. J Clin Psychiatry. 2009;70(7):1041-1050. PubMed doi:10.4088/JCP.08r04392
5. Melkersson KI, Hulting AL, Brismar KE. Different influences of classical antipsychotics and clozapine on glucose-insulin homeostasis in patients with schizophrenia or related psychoses. J Clin Psychiatry. 1999;60(11):783-791. PubMed doi:10.4088/JCP.v60n1112
6 Varma S, Bishara D, Besag FM, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships: therapeutic advances in psychopharmacology 2011;1(2):47-66.
7. Haring C, Fleischhacker WW, Schett P, et al. Influence of patient-related variables on clozapine plasma levels. Am J Psychiatry. 1990;147(11):1471-1475. PubMed
8. Mauri MC, Volonteri LS, Colasanti A, et al. Clinical pharmacokinetics of atypical antipsychotics: a critical review of the relationship between plasma concentrations and clinical response. Clin Pharmacokinet. 2007;46(5):359-388. PubMed doi:10.2165/00003088-200746050-00001
9. Bersani FS, Capra E, Minichino A, et al. Factors affecting interindividual differences in clozapine response: a review and case report. Hum Psychopharmacol. 2011;26(3):177-187. PubMed
10. Haring C, Meise U, Humpel C, et al. Dose-related plasma levels of clozapine: influence of smoking behavior, sex and age. Psychopharmacology (Berl). 1989;99(suppl 1):S38-S40. PubMed doi:10.1007/BF00442557
11. Bell R, McLaren A, Galanos J, et al. The clinical use of plasma clozapine levels. Aust N Z J Psychiatry. 1998;32(4):567-574. PubMed doi:10.3109/00048679809068332
12. Schulte P. What is an adequate trial with clozapine? therapeutic drug monitoring and time to response in treatment-refractory schizophrenia. Clin Pharmacokinet. 2003;42(7):607-618. PubMed doi:10.2165/00003088-200342070-00001
13. Hasegawa M, Gutierrez-Esteinou R, Way L, et al. Relationship between clinical efficacy and clozapine concentrations in plasma in schizophrenia: effect of smoking. J Clin Psychopharmacol. 1993;13(6):383-390. PubMed doi:10.1097/00004714-199312000-00003
14. Liu HC, Chang WH, Wei FC, et al. Monitoring of plasma clozapine levels and its metabolites in refractory schizophrenic patients. Ther Drug Monit. 1996;18(2):200-207. PubMed doi:10.1097/00007691-199604000-00015
15. Spina E, Avenoso A, Facciol× G, et al. Relationship between plasma concentrations of clozapine and norclozapine and therapeutic response in patients with schizophrenia resistant to conventional neuroleptics. Psychopharmacology (Berl). 2000;148(1):83-89. PubMed doi:10.1007/s002130050028
16. Ulrich S, Baumann B, Wolf R, et al. Therapeutic drug monitoring of clozapine and relapse—a retrospective study of routine clinical data. Int J Clin Pharmacol Ther. 2003;41(1):3-13. PubMed doi:10.5414/CPP41003
17. Greenwood-Smith C, Lubman DI, Castle DJ. Serum clozapine levels: a review of their clinical utility. J Psychopharmacol. 2003;17(2):234-238. PubMed doi:10.1177/0269881103017002014
18. Ghotbi R, Mannheimer B, Aklillu E, et al. Carriers of the UGT1A4 142T>G gene variant are predisposed to reduced olanzapine exposure: an impact similar to male gender or smoking in schizophrenic patients. Eur J Clin Pharmacol. 2010;66(5):465-474. PubMed doi:10.1007/s00228-009-0783-8
19. Lindroos K, Sigurdsson S, Johansson K, et al. Multiplex SNP genotyping in pooled DNA samples by a four-colour microarray system. Nucleic Acids Res. 2002;30(14):e70. PubMed doi:10.1093/nar/gnf069
20. Ronaghi M, Karamohamed S, Pettersson B, et al. Real-time DNA sequencing using detection of pyrophosphate release. Anal Biochem. 1996;242(1):84-89. PubMed doi:10.1006/abio.1996.0432
21. Ronaghi M, Uhlén M, Nyrén P. A sequencing method based on real-time pyrophosphate. Science. 1998;281(5375):363-365, 365. PubMed doi:10.1126/science.281.5375.363
22. Eckel RH, Alberti KG, Grundy SM, et al. The metabolic syndrome. Lancet. 2010;375(9710):181-183. PubMed doi:10.1016/S0140-6736(09)61794-3
23. Patteet L, Morrens M, Maudens KE, et al. Therapeutic drug monitoring of common antipsychotics. Ther Drug Monit. 2012;34(6):629-651. doi:10.1097/FTD.0b013e3182708ec5.. PubMed
24. Osby U, Olsson E, Edman G, et al. Psychotic disorder is an independent risk factor for increased fasting glucose and waist circumference. Nord J Psychiatry. 2014;68(4):251-258. PubMed
25. Diaz FJ, de Leon J, Josiassen RC, et al. Plasma clozapine concentration coefficients of variation in a long-term study. Schizophr Res. 2005;72(2-3):131-135. PubMed doi:10.1016/j.schres.2004.03.017
26. Bertilsson L, Carrillo JA, Dahl ML, et al. Clozapine disposition covaries with CYP1A2 activity determined by a caffeine test. Br J Clin Pharmacol. 1994;38(5):471-473. PubMed doi:10.1111/j.1365-2125.1994.tb04385.x
27. Rostami-Hodjegan A, Amin AM, Spencer EP, et al. Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: a predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients. J Clin Psychopharmacol. 2004;24(1):70-78. PubMed doi:10.1097/01.jcp.0000106221.36344.4d
28. Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917-1921. PubMed doi:10.2146/ajhp060414
29. Cheng YF, Lundberg T, Bondesson U, et al. Clinical pharmacokinetics of clozapine in chronic schizophrenic patients. Eur J Clin Pharmacol. 1988;34(5):445-449. PubMed doi:10.1007/BF01046700
30. Lane HY, Chang YC, Chang WH, et al. Effects of gender and age on plasma levels of clozapine and its metabolites: analyzed by critical statistics. J Clin Psychiatry. 1999;60(1):36-40. PubMed doi:10.4088/JCP.v60n0108
31. Mauri M, Volonteri LS, Fiorentini A, et al. Clinical outcome and plasma levels of clozapine and norclozapine in drug-resistant schizophrenic patients. Schizophr Res. 2004;66(2-3):197-198. PubMed doi:10.1016/S0920-9964(03)00159-2
32. Perera V, Gross AS, Polasek TM, et al. Considering CYP1A2 phenotype and genotype for optimizing the dose of olanzapine in the management of schizophrenia. Expert Opin Drug Metab Toxicol. 2013;9(9):1115-1137. PubMed
33. Ismail Z, Wessels AM, Uchida H, et al. Age and sex impact clozapine plasma concentrations in inpatients and outpatients with schizophrenia. Am J Geriatr Psychiatry. 2012;20(1):53-60. PubMed doi:10.1097/JGP.0b013e3182118318
34. Ackenheil M, Brפu H, Burkhart A, et al. Antipsychotic efficacy in relation to plasma levels of clozapine (author’s transl) [article in German]. Arzneimittelforschung. 1976;26(6):1156-1158. PubMed
35. Bowskill S, Couchman L, MacCabe JH, et al. Plasma clozapine and norclozapine in relation to prescribed dose and other factors in patients aged 65 years and over: data from a therapeutic drug monitoring service, 1996-2010. Hum Psychopharmacol. 2012;27(3):277-283. PubMed doi:10.1002/hup.2223
36. Swedish National Board of Health and Welfare. Swedish Prescribed Drug Register. http://www.socialstyrelsen.se/statistik/statistikdatabas/lakemedel. Accessed January 5, 2015.
37. Eap CB, Bender S, Jaquenoud Sirot E, et al. Nonresponse to clozapine and ultrarapid CYP1A2 activity: clinical data and analysis of CYP1A2 gene. J Clin Psychopharmacol. 2004;24(2):214-219. PubMed doi:10.1097/01.jcp.0000116646.91923.2f
38. Basson BR, Kinon BJ, Taylor CC, et al. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. J Clin Psychiatry. 2001;62(4):231-238. PubMed doi:10.4088/JCP.v62n0404
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top