ABSTRACT
Objective: To assess the benefits of anticonvulsant medications on benzodiazepine (BZD) use and delirium in patients with alcohol dependence at risk of alcohol withdrawal and admitted to the hospital without delirium.
Methods: This was a resident-led retrospective study of prospectively collected data for patients admitted to the monitored unit of a general medical ward between June 2016 and March 2017 for a variety of medical conditions. Patients were assigned to the usual care group (BZD as needed) or the intervention group (scheduled anticonvulsants and BZD as needed) based on admission census and order of arrival. Of 75 patients, 44 were assigned to the usual care group and 31 to the intervention group.
Results: Significantly lower BZD dosage (P = .0002) and lower Clinical Institute Withdrawal Assessment for Alcohol Scale-Revised scores were observed in the intervention group. Delirium occurred significantly less in the intervention group (0 versus 7 in the usual care group; P = .037).
Conclusions: Adjuvant anticonvulsant medications for alcohol withdrawal were efficacious in reducing BZD use, severity of symptoms of alcohol withdrawal, and occurrence of delirium in patients admitted to the general medical ward without delirium for reasons other than alcohol detoxification.
Prim Care Companion CNS Disord 2021;23(5):20m02860
To cite: Fargahi F, Shrestha R, Rawal H, et al. Impact of adjuvant anticonvulsant medications on benzodiazepine use and delirium in alcohol withdrawal syndrome. Prim Care Companion CNS Disord. 2021;23(5):20m02860.
To share: https://doi.org/10.4088/PCC.20m02860
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Medicine, MedStar Union Memorial Hospital, Baltimore, Maryland
*Corresponding author: Eskandar Alex Yazaji, MD, Department of Medicine, MedStar Union Memorial Hospital, 201 East University Pkwy, Baltimore, MD 21218 ([email protected]).
Alcohol withdrawal syndrome (AWS) is a serious complication in patients who have become chronically alcohol dependent and then undergo abrupt cessation. It is characterized by symptoms of autonomic hyperactivity such as agitation, tremors, and seizures. Symptoms usually develop in alcohol-dependent patients within 6 to 24 hours after the abrupt discontinuation or decrease of alcohol consumption. Delirium tremens, the most severe form of AWS in hospitalized patients, is characterized by features including hallucinations, disorientation, and tachycardia. In medical wards, delirium is associated with increased risk of mortality and morbidity by prolonging hospital stay, which can lead to increased risk of poor outcome including hospital-acquired infection and poor physical and cognitive recovery.1 Management of severe alcohol withdrawal requires intensive care unit admission.2 Mild alcohol withdrawal usually does not require medical management. However, management of less severe alcohol withdrawal is challenging in patients with multiple medical comorbidities admitted to the medical ward.3–7
AWS is usually caused by reduced γ-aminobutyric acid (GABA) activity and N-methyl-d-aspartate (NMDA) glutamate overactivity.8 Benzodiazepines (BZDs) are the preferred agents for managing AWS.9 However, this class of drug is associated with a high risk of cognitive impairment and delirium.10 Anticonvulsant medications have been shown to be as effective as lorazepam in reducing withdrawal symptoms in patients with mild to moderate AWS,11,12 while producing less amnesia and fewer sedative effects.13 Gabapentin increases GABA concentrations via interaction with the α2δ subunit of voltage-dependent calcium channels and by direct synthesis, and it also has been shown to reduce withdrawal excitability in hippocampal slices.14,15 Valproic acid and derivatives act to enhance brain levels of GABA, suppressing glutamate function via NMDA receptors and suppressing the withdrawal reaction.16 Patients treated with divalproex sodium have had lower requirements for BZDs and less progression in withdrawal symptom severity.17,18 Patients with more severe AWS have a relatively low response to antiepileptic medications alone,17,19 but gabapentin and divalproex have shown efficacy in reducing withdrawal symptoms in patients with less severe AWS.15,17 Gabapentin has been shown to be effective as an adjunct with BZDs in severe AWS.20
We speculated that adjuvant use of anticonvulsant medications with BZDs for AWS would reduce the BZD use and adverse effects in patients with mild to moderate AWS. In collaboration with our inpatient pharmacy, we developed a protocol for patients with mild to moderate AWS that included administration of adjuvant anticonvulsive medications with BZD for use in the monitored unit of the general medical ward.
METHODS
This study was approved by our institutional review board. One investigator (F.F.) retrospectively reviewed charts of prospectively collected data for patients with multiple comorbidities with a diagnosis other than alcohol detoxification admitted to our institution from June 2016 to March 2017.
All patients presented to the hospital emergency department, and initial eligibility screening was performed upon admission. Inclusion criteria included age 18 years or older, alcohol dependence, and no delirium on admission based on a negative Confusion Assessment Method (CAM).21 Participants reported an estimated amount of daily alcohol use and the number of previous medicated withdrawals and alcohol withdrawal delirium episodes. Participants were asked about medications used before admission that might confound or complicate alcohol withdrawal treatment, such as opioids, gabapentin, valproic acid, or BZDs. Exclusion criteria were delirium at presentation to the emergency department judged by negative CAM, any psychiatric condition requiring stabilization, pregnancy or nursing, severe cognitive deficit, or current use of BZD or anticonvulsant medications. A focused medical and psychiatric history, physical examination, and CAM assessment were done for all participants at admission.
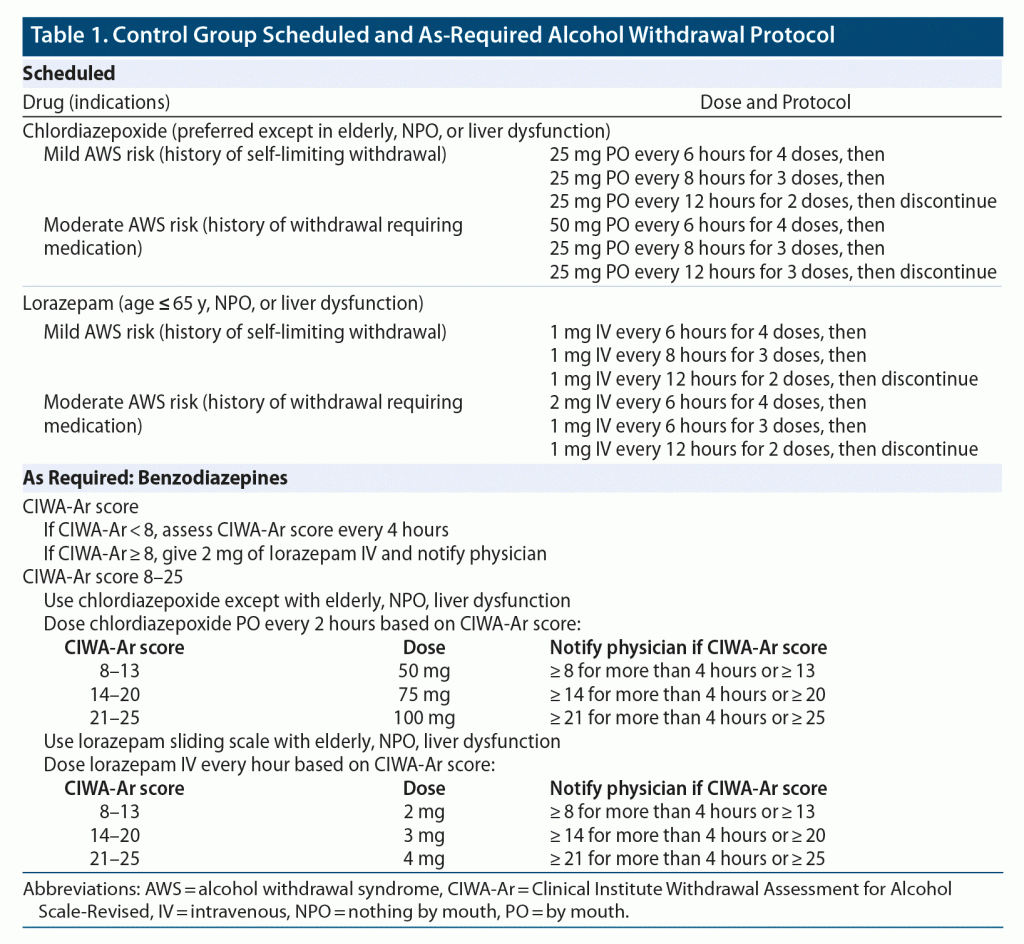
In the emergency department, all patients were started on the hospital standard BZD protocol for alcohol withdrawal prophylaxis based on a standard Clinical Institute Withdrawal Assessment for Alcohol Scale-Revised (CIWA-Ar)22 assessment. In patients with a CIWA-Ar score < 8, nurses assessed patients every 4 hours for CIWA-Ar score and did not administer BZDs. Patients with CIWA-Ar scores between 8 and 25 underwent CIWA-Ar assessment every 2 hours and were treated with chlordiazepoxide or lorazepam based on their age and liver function and whether they could tolerate oral medication. Lorazepam-equivalent dose was used to calculate BZD dosage and duration requirements.23
Patient Allocation
our institutional protocol, patients with a CIWA-Ar score > 25 were managed in the intensive care unit and were not included in our study. Of 75 patients included, 44 were assigned to the usual care group and 31 to the intervention group based on order of admission. The medical team for each group consisted of an attending and 3 medical residents. Patients in both arms were located on the same medical ward unit. One study investigator (F.F.) provided education to the medical team members at the start of their rotation in the medical ward.
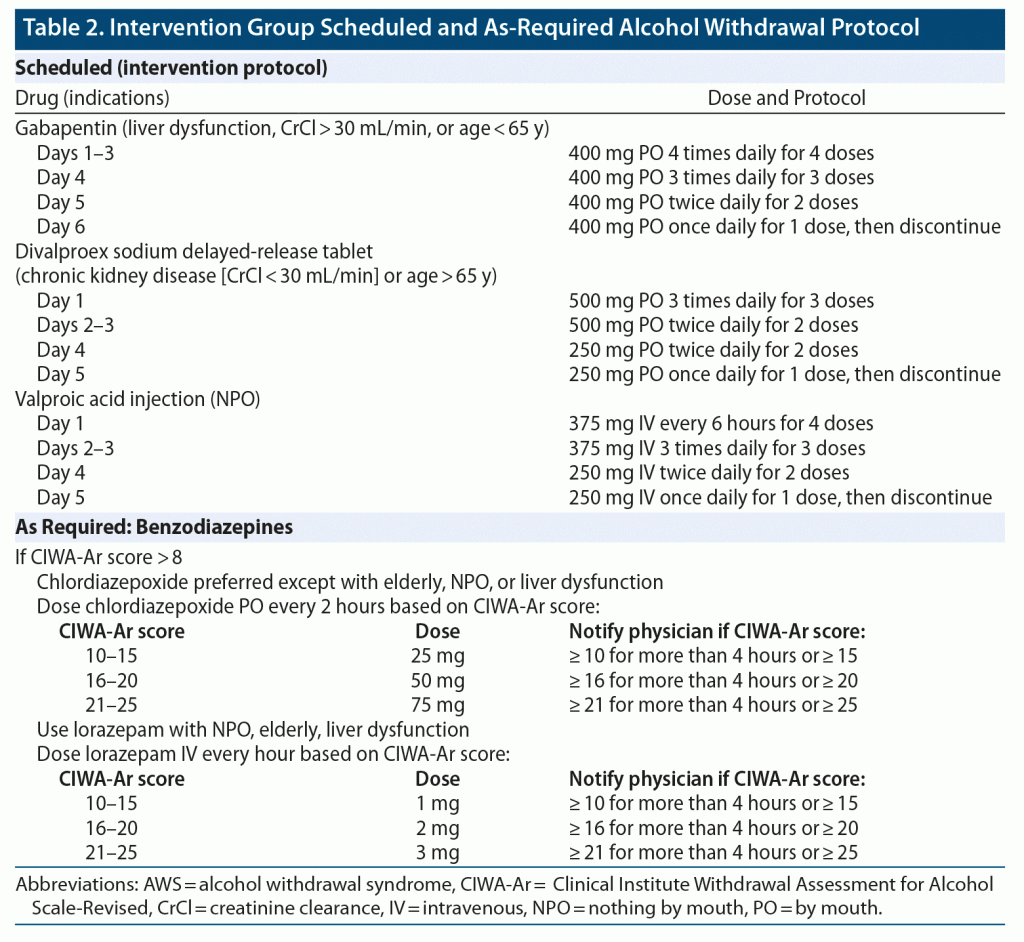
The control and intervention protocols are shown in Table 1 and Table 2. The intervention protocol included standing doses of an anticonvulsant medication, regardless of the patient’s CIWA-Ar score. Patients in the intervention group received gabapentin, divalproex, or valproic acid based on age, kidney function, and whether the patient could tolerate oral medication. Chlordiazepoxide and lorazepam were used as a rescue medication only if patients had a CIWA-Ar score > 8–10. In addition to the protocol medications, all participants received a daily oral multivitamin, folic acid, and thiamine. If indicated, prochlorperazine was given for nausea. Intravenous metoprolol was administered for systolic blood pressure (SBP) > 160 mm Hg and/or heart rate > 120 bpm, and haloperidol was given for hallucinations, agitation, or disorientation. Daily assessment included a brief examination to document mental status and CAM assessment.
The dosage and duration of BZD use and occurrence of delirium was compared between the control and intervention groups. Safety was assessed based on adverse clinical events.
Statistical Analysis
Descriptive statistics used mean and standard deviation for continuous variables and median with interquartile ranges (IQRs) for continuous variables with skewed distribution. Frequencies and percentages were analyzed for categorical variables. Comparison between groups of categorical variables was conducted using χ2 test and Fisher exact test for small expected cell sizes. Comparison of continuous variables between 2 groups was conducted using 2-sample t test and Wilcoxon rank sum test when variables had skewed distribution.
Multivariable regression analysis was conducted for BZD amount before admission, BZD amount after admission, and BZD duration after admission controlling for age, race, and sex. Due to the large number of patients with zero values for BZD amount and duration, regression was conducted using a zero inflated negative binomial model with 2 separate parts. The first part modeled zeros (likelihood of not receiving BZD), and the second modeled amount and duration of BZD among patients who received BZD. Multivariable linear regression analysis controlling for age, sex, and race was conducted for mean severity of CIWA-Ar score before and after admission. Mean CIWA-Ar score before admission was skewed to the right. Therefore, regression analysis was performed on log-transformed mean CIWA-Ar score. Results were considered significant at the α < .05 level. Analyses were conducted using SAS 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
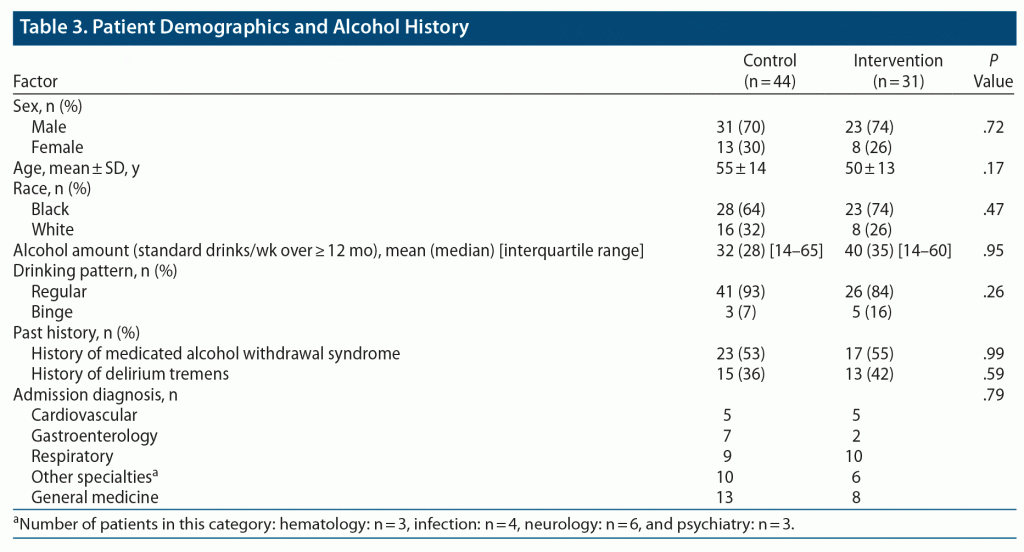
The control group (n = 44) and the intervention group (n = 31) were similar in demographics and in number of daily standard drinks or drinking pattern, either regular or binge drinking (Table 3). Fifty-four (72%) of the participants were male, and the majority were Black (68%). There was no significant difference between the 2 treatment groups in history of alcohol withdrawal, delirium tremens, and drinking pattern, either regular or binge pattern.
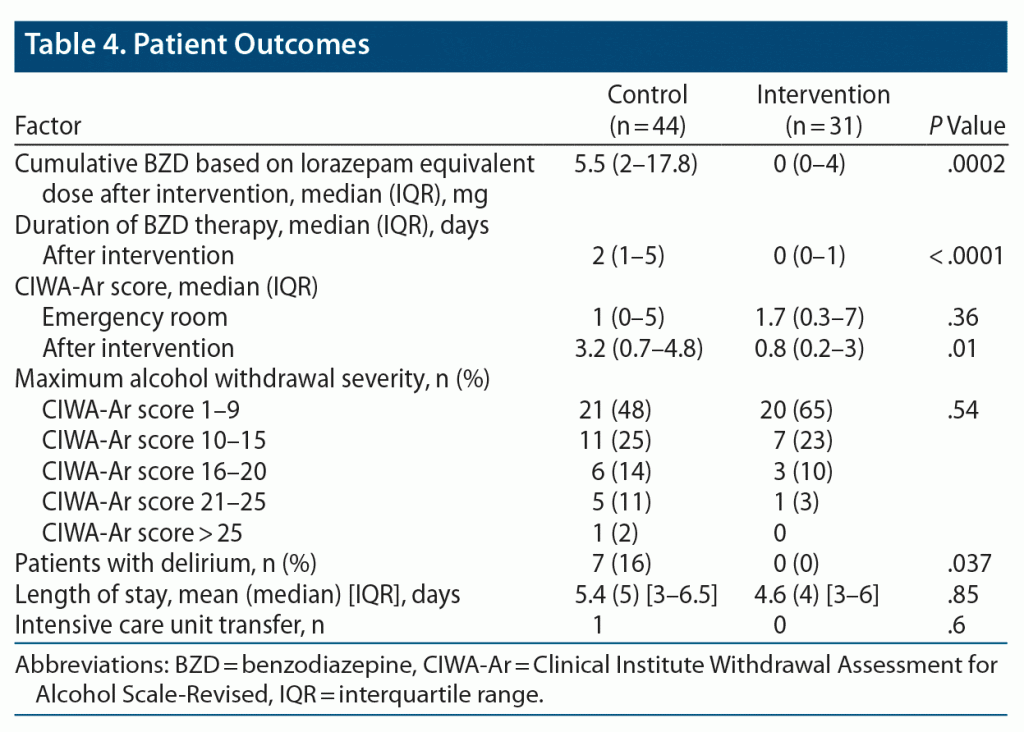
Patients in the intervention group received gabapentin (24 patients, 77%), divalproex sodium (3 patients, 10%), or valproic acid (4 patients, 13%). The intervention group had significantly lower as-needed BZD requirement than the control group. The median cumulative BZD dosage was 0 mg (IQR = 0–4) for the intervention group compared to 5.5 mg (IQR = 2–17.8) for the control group (P = .0002) (Table 4). Patients in the intervention group had significantly lower CIWA-Ar score (median = 0.8 [IQR = 0.2–3]) compared to the control group (median = 3.2 [IQR = 0.7–4.8]) (P = .01). After admission, the mean CIWA-Ar score was 89% lower in the intervention group than in the control group (P = .0021). Occurrence of delirium was significantly lower in the intervention group with 0 patients with delirium compared with 7 (16%) participants in the control group (P = .037). No difference in hospital length of stay or intensive care unit transfer was found between the groups. No adverse events were associated with gabapentin, valproic acid, or divalproex, and no medications had to be discontinued.
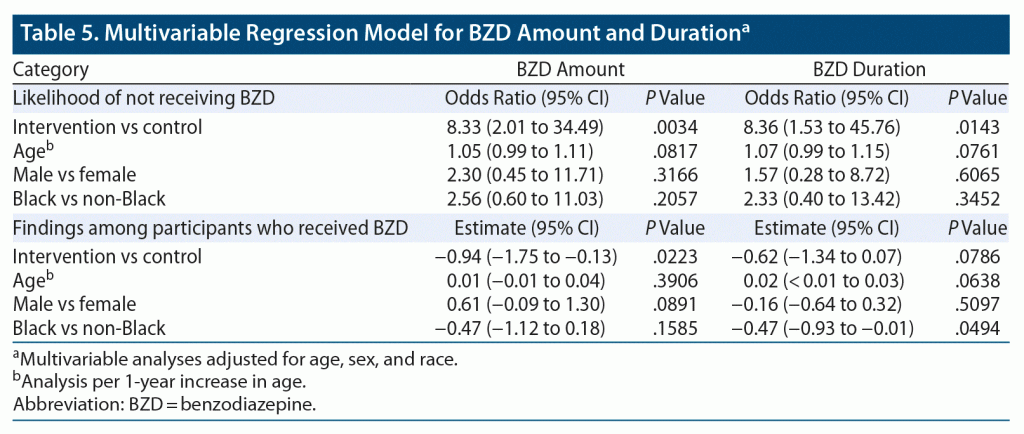
Multivariable regression analysis showed no significant difference in BZD requirements before admission between the 2 groups. After admission, patients in the control group were more likely to receive BZD (odds ratio = 8.33, P = .0034). Among patients who received BZDs after admission, those in the intervention group received a mean lorazepam-equivalent dose of 3 mg, which was a 61% smaller dosage amount compared to the control group who received 14 mg (P = .0223). There was no significant difference in number of days of BZD therapy after admission between the control and intervention groups (P = .0786). However, the duration of BZD treatment was significantly shorter for Black patients than for non-Black patients in the intervention group. Black patients, on average, had 37% shorter duration of BZD treatment than non-Black patients (P = .0495) (Table 5).
DISCUSSION
In this study, adjuvant anticonvulsive therapy reduced BZD use, occurrence of delirium, and severity of withdrawal symptoms in complex patients with medical problems other than detoxification who were at risk of AWS. The current findings suggest that a protocol incorporating adjuvant anticonvulsive therapy is effective in reducing BZD dosage and adverse effects in the treatment of mild to moderate AWS.
Gabapentin has been shown to be safe and can be easily administered orally. Valproic acid was chosen because it could be administered intravenously and has a safe profile in elderly patients and in patients with renal impairment. Although these agents have a slightly different mechanism of action, they can be used as adjunctive therapy to traditional benzodiazepines because these medications have effects on glutamate and GABA neurotransmission.
Anticonvulsant medications may be used in place of BZDs in mild cases of alcohol withdrawal (ie, CIWA-Ar < 8).24–26 This study supports previous findings of effectiveness of non-BZD anticonvulsant compounds in the treatment of alcohol-dependent patients. Among this group of medications, gabapentin has been shown to be safe in this patient population27 and to have beneficial effects in AWS.28–30 Compared with BZD, gabapentin has lower abuse potential and no risk of dependence.27 Voris et al15 analyzed the effects of gabapentin in the treatment of AWS for 17 participants in an inpatient setting and 25 outpatients in a retrospective study. Both sets of data suggested that gabapentin works well in treatment of mild to moderate alcohol withdrawal. Levine et al20 showed that initiation of high-dose gabapentin (≥ 1,800 mg/d) during the first 48 hours of hospital admission was associated with a significant reduction in BZD exposure, faster stabilization of alcohol withdrawal–related symptoms, and shorter hospital length of stay in patients presenting to the emergency department with a diagnosis of severe AWS.
As shown in our study, valproic acid is also an effective treatment for alcohol withdrawal, along with valproate and divalproex, which are metabolized to valproic acid. Valproic acid and derivatives are reported to be associated with a reduced need for rescue medications including BZD and have been shown to be efficacious in faster reduction of AWS symptoms.17,31,32 A study by Longo et al32 demonstrated that valproate was associated with a more rapid and consistent reduction of AWS compared to BZD. In another study, Reoux et al17 showed possible use of valproic acid in the treatment of AWS.
Limitations
We did not include patients admitted to the intensive care unit for severe AWS. Patients were not randomized, but our study groups were similar and data were prospectively collected. This was a single-center study with a relatively small population, but we were able to show significant benefit of adjuvant anticonvulsive therapy in this group of patients. Our results reflect findings in our medical-surgical step-down unit, but they might not extrapolate to other institutions.
CONCLUSION
In this quality improvement project, adjuvant anticonvulsant medications for alcohol withdrawal were efficacious in reducing BZD use, occurrence of delirium, and severity of symptoms of alcohol withdrawal in complex patients with medical problems other than detoxification who were at risk of AWS. The findings suggest that adjuvant anticonvulsive therapy might be considered in treatment protocols in this setting.
Submitted: November 6, 2020; accepted February 9, 2021.
Published online: October 21, 2021.
Potential conflicts of interest: None.
Funding/support: Internal institutional funding from Graduate Medical Education (GME) MedStar Health, MedStar Union Memorial Hospital.
Role of the sponsor: MedStar Health, MedStar Union Memorial Hospital was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of data; or preparation, review, or approval of the manuscript
Acknowledgments: The authors thank Sameer Desale, MS, and Mihriye Mete, PhD, of MedStar Health Research Institute, Baltimore, Maryland, for statistical support and Lyn Camire, MA, ELS, of MedStar Union Memorial Hospital, Baltimore, Maryland, for editorial assistance. Mr Desale, Dr Mete, and Ms Camire report no conflicts of interest related to the subject of this article.
Clinical Points
- Using the reported combined treatment protocol, clinicians may be able to help limit benzodiazepine use and risk of delirium in patients with mild to moderate alcohol withdrawal syndrome admitted to the general medical ward with a diagnosis other than alcohol detoxification.
- Anticonvulsant medications used adjunctively with benzodiazepines appear to represent an important tool for hospital physicians treating patients with alcohol withdrawal syndrome.
References (32)

- McCusker J, Cole M, Dendukuri N, et al. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med. 2003;18(9):696–704. PubMed CrossRef
- Mirijello A, D’Angelo C, Ferrulli A, et al. Identification and management of alcohol withdrawal syndrome. Drugs. 2015;75(4):353–365. PubMed CrossRef
- Gupta NM, Lindenauer PK, Yu PC, et al. Association between alcohol use disorders and outcomes of patients hospitalized with community-acquired pneumonia. JAMA Netw Open. 2019;2(6):e195172. PubMed CrossRef
- Akano EO, Otite FO, Chaturvedi S. Alcohol withdrawal is associated with poorer outcome in acute ischemic stroke. Neurology. 2019;93(21):e1944–e1954. PubMed CrossRef
- de Roux A, Cavalcanti M, Marcos MA, et al. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129(5):1219–1225. PubMed CrossRef
- Saitz R, Ghali WA, Moskowitz MA. The impact of alcohol-related diagnoses on pneumonia outcomes. Arch Intern Med. 1997;157(13):1446–1452. PubMed CrossRef
- de Wit M, Jones DG, Sessler CN, et al. Alcohol-use disorders in the critically ill patient. Chest. 2010;138(4):994–1003. PubMed CrossRef
- Bayard M, McIntyre J, Hill KR, et al. Alcohol withdrawal syndrome. Am Fam Physician. 2004;69(6):1443–1450. PubMed
- Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348(18):1786–1795. PubMed CrossRef
- Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. PubMed CrossRef
- Bonnet U, Hamzavi-Abedi R, Specka M, et al. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol. 2010;45(2):143–145. PubMed CrossRef
- Mariani JJ, Rosenthal RN, Tross S, et al. A randomized, open-label, controlled trial of gabapentin and phenobarbital in the treatment of alcohol withdrawal. Am J Addict. 2006;15(1):76–84. PubMed CrossRef
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the alcohol withdrawal syndrome. Cochrane Database Syst Rev. 2011;(6):CD008537. PubMed CrossRef
- Bailey CP, Molleman A, Little HJ. Comparison of the effects of drugs on hyperexcitability induced in hippocampal slices by withdrawal from chronic ethanol consumption. Br J Pharmacol. 1998;123(2):215–222. PubMed CrossRef
- Voris J, Smith NL, Rao SM, et al. Gabapentin for the treatment of ethanol withdrawal. Subst Abus. 2003;24(2):129–132. PubMed CrossRef
- Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16(10):669–694. PubMed CrossRef
- Reoux JP, Saxon AJ, Malte CA, et al. Divalproex sodium in alcohol withdrawal: a randomized double-blind placebo-controlled clinical trial. Alcohol Clin Exp Res. 2001;25(9):1324–1329. PubMed CrossRef
- Reoux JP, Saxon AJ, Shen D. Pharmacokinetic profile of an oral loading dose of divalproex sodium during acute alcohol withdrawal. J Clin Psychopharmacol. 2006;26(1):105–107. PubMed CrossRef
- Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome: a focus on non-benzodiazepine GABAergic medications. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1106–1117. PubMed CrossRef
- Levine AR, Carrasquillo L, Mueller J, et al. High-dose gabapentin for the treatment of severe alcohol withdrawal syndrome: a retrospective cohort analysis. Pharmacotherapy. 2019;39(9):881–888. PubMed CrossRef
- Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the Confusion Assessment Method: a new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. PubMed CrossRef
- Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA-Ar). Br J Addict. 1989;84(11):1353–1357. PubMed CrossRef
- Miller NS, Gold MS. Management of withdrawal syndromes and relapse prevention in drug and alcohol dependence. Am Fam Physician. 1998;58(1):139–146. PubMed
- Hammond CJ, Niciu MJ, Drew S, et al. Anticonvulsants for the treatment of alcohol withdrawal syndrome and alcohol use disorders. CNS Drugs. 2015;29(4):293–311. PubMed CrossRef
- Leung JG, Hall-Flavin D, Nelson S, et al. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897–906. PubMed CrossRef
- Lum E, Gorman SK, Slavik RS. Valproic acid management of acute alcohol withdrawal. Ann Pharmacother. 2006;40(3):441–448. PubMed CrossRef
- Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221–227. PubMed CrossRef
- Myrick H, Malcolm R, Brady KT. Gabapentin treatment of alcohol withdrawal. Am J Psychiatry. 1998;155(11):1632. PubMed CrossRef
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514–519. PubMed CrossRef
- Bozikas V, Petrikis P, Gamvrula K, et al. Treatment of alcohol withdrawal with gabapentin. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):197–199. PubMed CrossRef
- Lambie DG, Johnson RH, Vijayasenan ME, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J Psychiatry. 1980;14(3):213–215. PubMed CrossRef
- Longo LP, Campbell T, Hubatch S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;21(2):55–64. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top