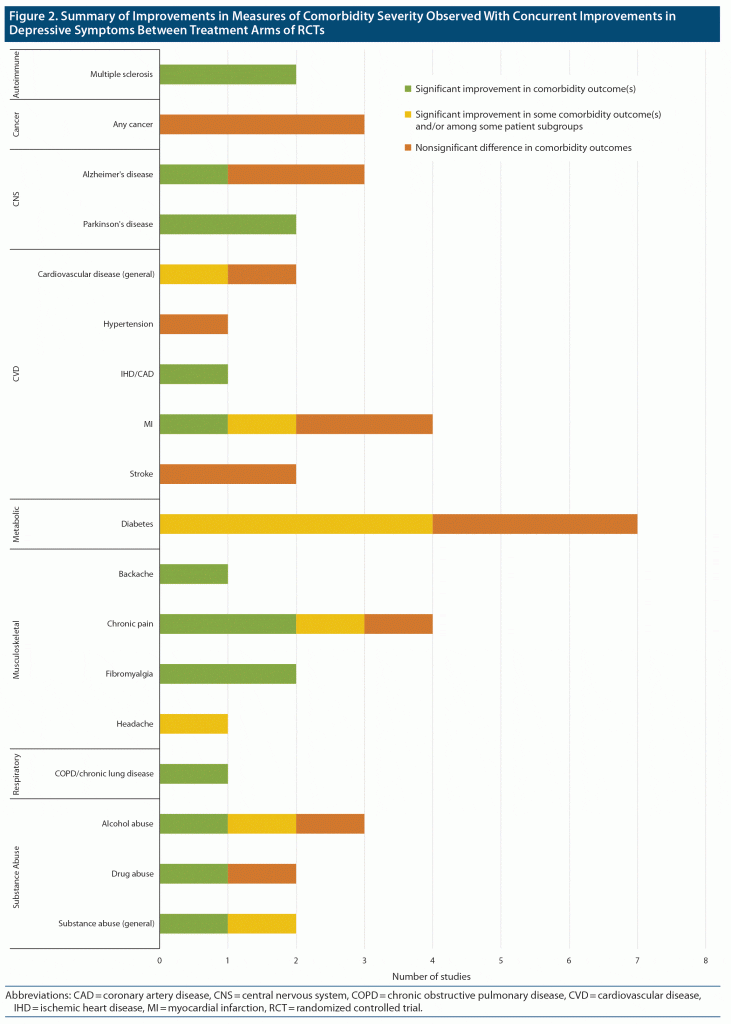ABSTRACT
Objective: To identify and summarize data that describe the impact of effectively treating major depressive disorder (MDD) on the severity or risk of serious comorbidities.
Data Sources: MEDLINE, Embase, PsycINFO, Cochrane Database of Systematic Reviews, and several congresses were searched. Searches included terms related to MDD, randomized controlled trials (RCTs), and physical comorbidities and were restricted to English-language publications. Searches were conducted in November 2019 for the previous 2 years for conference proceedings; no date restriction was applied to the database searches.
Study Selection: Included studies were RCTs or meta-analyses that assessed depression therapies. Studies were required to report a statistically significant improvement in depression scores as well as the concurrent impact on comorbidities. A total of 1,997 articles were initially identified for screening.
Data Extraction: Two investigators extracted data and assessed study quality.
Results: A total of 30 studies, including 24 RCTs (N = 6,333) and 6 meta/pooled analyses of RCTs, were included. Findings in several comorbidity categories were mixed; for example, in half (4 of 8) of the identified studies in people with cardiovascular disease and depression, individuals who received treatment leading to reduced depressive symptoms compared with a control arm also had a significantly decreased incidence of cardiovascular events or significantly improved cardiac disease symptom/severity scores compared with controls. Significant improvements in comorbid disease severity observed alongside improvements in depressive symptoms were also noted in studies of comorbid Parkinson’s disease, multiple sclerosis, chronic pain and fibromyalgia, and chronic obstructive pulmonary disease.
Conclusions: Effective treatment of MDD may lead to a reduction in the severity of certain serious comorbidities. These results highlight the importance of appropriate and timely treatment of MDD.
Prim Care Companion CNS Disord 2023;25(1):22r03330
To cite: Arnaud AM, Brister TS, Duckworth K, et al. Impact of treating depression on associated comorbidities: a systematic literature review. Prim Care Companion CNS Disord. 2023;25(1):22r03330.
To share: https://doi.org/10.4088/PCC.22r03330
© 2023 Physicians Postgraduate Press, Inc.
aSage Therapeutics, Inc, Cambridge, Massachusetts
bNational Alliance on Mental Illness, Arlington, Virginia
cDepression and Bipolar Support Alliance, Chicago, Illinois
dMental Health America of Ohio, Columbus, Ohio
eTantalus Medical Communications Ltd, Victoria, British Columbia, Canada
*Corresponding author: Ellison D. Suthoff, MBA, 215 First St, Cambridge, MA 02142 ([email protected]).
Many physical and psychiatric comorbidities have been associated with major depressive disorder (MDD).1 The association between mental health and physical health is often described as bidirectional in nature; while in certain cases depression may develop following an initial underlying disease,2 there is also evidence that individuals may be at a greater risk of developing various comorbidities because of depression (or existing comorbidities may be worsened by the existence of comorbid depression).1 Recent research identified significant associations between depression and the incidence and/or worsening of a broad range of comorbidities in a systematic review of observational studies.3 Furthermore, an analysis of over 9 million commercially insured people in the United States who were diagnosed with MDD in 2016 showed that 85% had at least 1 comorbidity, and nearly 30% had 4 or more comorbidities.4
The humanistic and economic burden of comorbidities associated with depression is considerable. An analysis of US Medical Expenditure Panel Survey data (2010–2015) found that approximately 60% of individuals with MDD and/or any anxiety disorder had at least 1 comorbid chronic noncommunicable disease, which led to increased annual health care costs of up to nearly $4,000 per person with MDD that was driven primarily by cardiovascular disease (CVD) and pain conditions.5 The presence of comorbid disease also led to significant decreases in measures of physical patient-reported quality of life among these affected individuals.5
Although several studies have demonstrated that depression is often accompanied by the presence of comorbidities or worsening of existing comorbidities, it remains unclear whether effective treatment of MDD could improve the incidence risk or severity of comorbid diseases. The objective of this systematic literature review was to identify and summarize data that evaluates the impact of effectively treating MDD on the risk or severity of comorbidities known to be associated with depression.
METHODS
A systematic literature search for studies that examined the association between improvement in MDD and the concurrent impact on comorbidities was conducted using methods consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines.6
The methods of determining comorbidities have been described previously.3 Briefly, categories of comorbidities were determined from preliminary research, advocacy group reports, medical claims data, and expert opinion. The final list of comorbidity categories included cancer, central nervous system (CNS) disorders, CVD, metabolic and endocrine diseases, autoimmune and gastrointestinal diseases, pain-related conditions, respiratory disorders, and substance abuse disorders.
Databases searched included Embase, MEDLINE (including MEDLINE In-Process), Cochrane Database of Systematic Reviews, Cochrane Controlled Register of Trials, and PsycINFO, and the search used terms for MDD, comorbidity categories, and randomized controlled trials (RCTs; the full search strategy is provided in Supplementary Table 1). In addition, abstracts from several relevant congresses were reviewed, and hand searches of referenced publications were undertaken. Searches were conducted in November 2019 for the previous 2 years for conference proceedings; no date restriction was applied to the database searches.
Included studies were required to report a statistically significant improvement (ie, reduction) in depressive symptoms following any depression treatment intervention compared with another treatment or control arm. Studies were also required to assess change in either comorbidity incidence (ie, risk of developing a downstream comorbidity) or the disease severity of existing comorbidities over the same treatment period. Included studies were required to be RCTs (including meta-analyses of randomized trials), and only English-language records were searched; a complete list of criteria is provided in Supplementary Table 2.
Search results were screened by 2 separate reviewers (M.L.R. and I.A.) initially by titles and abstracts, followed by a review of the full text; any disputes were resolved through discussion between reviewers or consultation with a third reviewer. Data from included studies were extracted by 2 independent reviewers (M.L.R. and I.A.), and any discrepancies between extractions were verified for accuracy by an independent third reviewer. Data describing the study methodology, participant demographic and clinical characteristics, and changes in MDD and comorbidity outcomes were extracted. The quality of included studies was assessed by reviewers using the checklists recommended by the National Institute for Health and Care Excellence for RCTs and meta-analyses (see Supplementary Tables 3–5).7,8
RESULTS
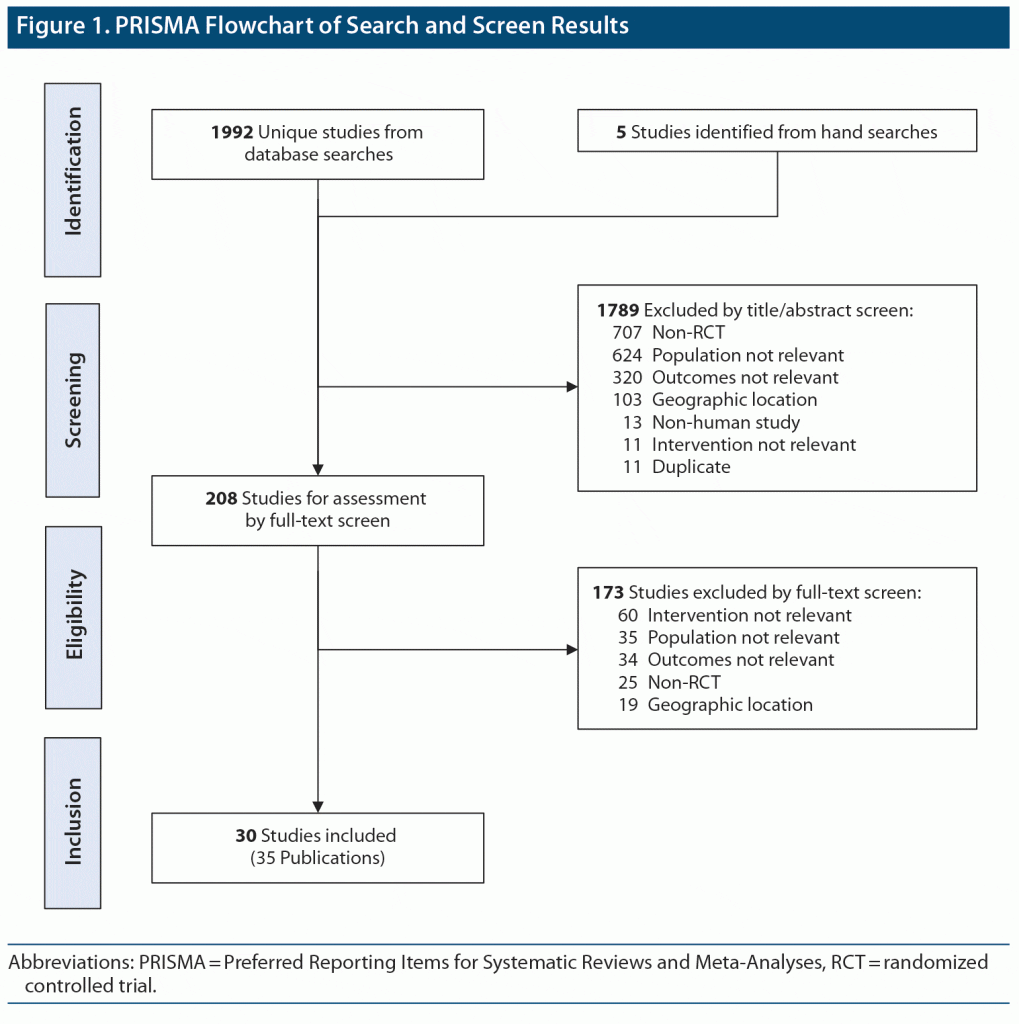
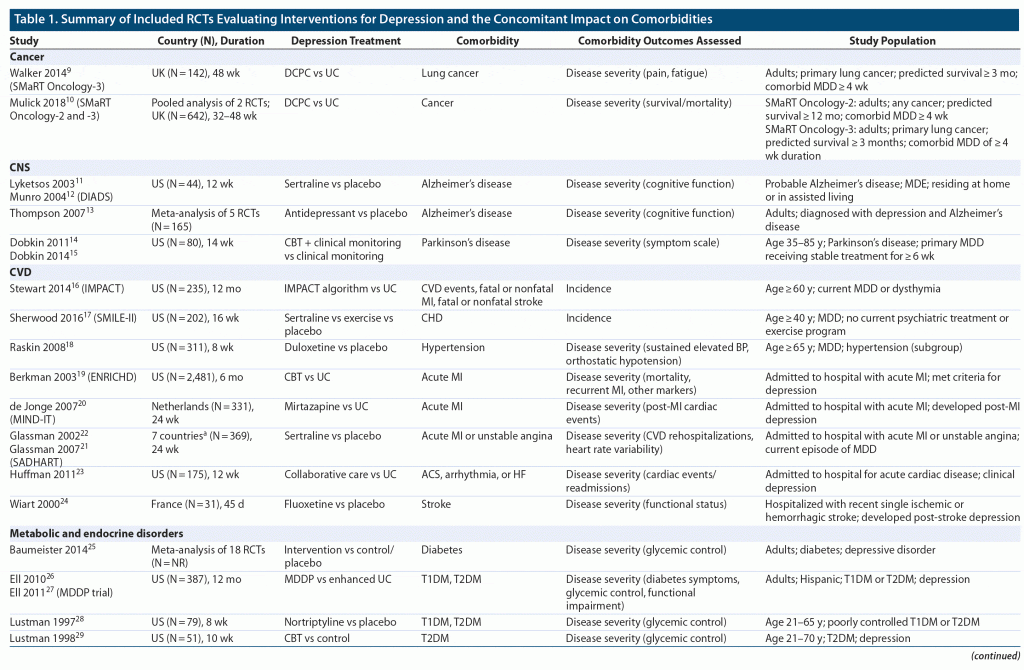
In total, 1,992 articles were identified for screening from the database searches for initial screening, which were combined with another 5 relevant articles from conference abstracts and hand searches. The systematic review included 30 studies from 35 publications, including 24 RCTs (N = 6,333) and 6 meta-analyses or pooled analyses of RCTs (Figure 1). Included studies included treatment with several nondrug interventions, such as cognitive-behavioral therapy (CBT), as well as various pharmacologic therapies. Most studies were under 1 year in duration, with many less than 6 months. A description of included studies is provided in Table 1,9–43 and a summary of findings for changes in comorbidity severity is shown in Figure 2.
Cancer
A total of 2 RCTs were identified that assessed the impact of improving depressive symptoms on outcomes in people with cancer: the Symptom Management Research Trials (SMaRT) Oncology-2 and -3 studies.9,10 In general, changes in participant survival or pain scores were not observed alongside improvements in depressive symptoms.
The included studies9,10 were both UK-based trials that compared a novel depression care program (Depression Care for People with Cancer; DCPC) to usual care among 2 cohorts: those with a good prognosis (SMaRT Oncology-2; any cancer [N = 500]) or poor prognosis (SMaRT Oncology-3; lung cancer only [N = 142]). Although the DCPC program led to a significantly greater proportion of individuals with a depression treatment response compared with usual care in both cohorts (62% vs 17%; P < .0001 for SMaRT Oncology-2; odds ratio [OR]: 5.88; 95% confidence interval [CI], 2.42–14.33; P < .0001 for SMaRT Oncology-3), there were no significant changes observed between groups in overall survival or pain symptoms on a 100-point visual analog scale (VAS; reported in SMaRT Oncology-3 only).9,10 Between-group changes in mean fatigue symptoms, measured using a 100-point VAS, were numerically lower in the DCPC group compared with usual care in an analysis that approached significance (59.3 vs 64.1; P = .058; SMaRT Oncology-3 only).9
Using survival as a surrogate endpoint for disease severity, both trials reported that 6-year survival was not significantly different between groups (72% for DCPC vs 69% for usual care in SMaRT Oncology-2; 23% vs 15% in SMaRT Oncology-3).10
CNS Disorders
The review identified 1 RCT that met inclusion criteria, the Depression in Alzheimer’s Disease Study (DIADS),11,12 and 1 meta-analysis of 5 RCTs,13 which collectively assessed depressive symptom improvement in conjunction with changes in Alzheimer’s disease severity. Overall, these studies did not demonstrate changes in measures of Alzheimer’s disease severity in conjunction with depressive symptom improvement. In addition, a single RCT was identified that assessed improvement in depressive symptoms following CBT alongside changes in Parkinson’s disease severity; this study showed that many improvements in disease severity scores occurred alongside improvements in depressive symptoms.14,15
In DIADS, 44 people with Alzheimer’s disease and major depressive episode were randomized to 12 weeks of treatment with sertraline or placebo.11 Overall, a significantly greater proportion of participants achieved depression response criteria following treatment with sertraline compared with placebo (38% full and 46% partial vs 20% full and 15% partial, respectively; P = .007).11 Mean 12-week scores for the Cornell Scale for Depression in Dementia (10.3 vs 14.9) and Hamilton Depression Rating Scale (HDRS) (13.2 vs 17.3) were also significantly improved in the sertraline-treated group compared with placebo (P = .011).11 However, mean Alzheimer’s disease severity scores at week 12, including daily living impairment, behavior disturbance, and cognition, were not significantly different in sertraline-treated individuals compared with those who received placebo.11 A further analysis of changes in mean scores from various cognitive tests similarly showed no differences between the sertraline and placebo groups.12 When participants were analyzed based on depression treatment response (regardless of randomized treatment group), those with improved mood did not show a significant change in cognitive scores compared with participants who did not have an improved mood.12
Similar to the findings from DIADS, the meta-analysis13 (N = 165) reported significant improvements in antidepressant-treated participants compared with placebo for depression response (OR: 2.32; 95% CI, 1.04–5.16; P = .04) and remission (OR: 2.75; 95% CI, 1.13–6.65; P = .03), whereas the weighted mean difference in Mini-Mental State Examination (MMSE) score was not significant between groups (−0.71; 95% CI, −3.20 to 1.79; P = .58).
The impact of 10-week treatment with CBT and clinical monitoring compared with clinical monitoring alone was evaluated in an RCT of 80 people with Parkinson’s disease and comorbid MDD.14,15 In this study, participants randomized to CBT showed significant improvements in HDRS score (mean score: 13.58 vs 19.33; P < .0001) and Beck Depression Inventory (BDI) score (mean score: 9.74 vs 17.45; P = .001) compared with those who received clinical monitoring only at end of treatment (week 10) and also demonstrated an improvement in mean Unified Parkinson’s Disease Rating Scale scores in the same comparison (40.11 vs 49.59; P = .001).14 The study additionally examined the impact of depression treatment (regardless of intervention group) on various neuropsychological tests, showing that participants who experienced significant depressive symptom improvement also had concurrent improvements in verbal memory (assessed with total recall [P = .009] and recognition tests [P = .007]) and executive functioning based on inhibition score (P = .02).15 Furthermore, a stepwise regression model controlling for baseline scores of Parkinson’s disease severity, education, and age showed that depressive symptom improvement contributed unique variance to verbal memory measures of total recall (6%; P = .009) and recognition (11%; P = .001) and to the executive functioning measure of inhibition (2%; P = .021).15
Cardiovascular Disease
A total of 8 RCTs (9 references) were identified that assessed the impact of depression interventions on improvement in depressive symptoms and the concurrent change in CVD-associated comorbidities. These included 2 RCTs assessing the risk of incident CVD or changes in coronary artery disease/coronary heart disease (CHD) risk factors,16,17 1 RCT assessing the impact on existing hypertension,18 4 RCTs assessing the impact on subsequent cardiac events in people admitted to the hospital with acute cardiac disease,19–23 and 1 RCT assessing disease severity in people with stroke who developed poststroke depression.24 Many studies showed that people with cardiac disease who experienced improvements in depressive symptoms also had significantly fewer cardiovascular events and improvements in disease severity scores; however, results were not consistent across all trials.
The 2 studies assessing how CVD incidence/risk factors were affected when depressive symptoms improved included the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) study (N = 235) and the Standard Medical Intervention versus Long-term Exercise (SMILE-II) study (N = 202).16,17 In IMPACT, participants were randomized to receive a stepped-care algorithm using both antidepressant and behavioral therapy (IMPACT group) or usual care for 12 months, with a follow-up period for CVD events of 8 years. Among people without baseline CVD, the IMPACT group showed a significant improvement in the 20-item Symptom Checklist Depression Scale (SCL-20) depression scores compared with usual care (mean change from baseline: −0.4 vs 0.1; P < .001) as well as lower rates of combined CVD events (28% vs 47%; P = .010; HR: 0.52; 95% CI, 0.31–0.86; P < .05).16 When individual events were examined among participants with no baseline CVD, there was no significant association between treatment effect and risk of myocardial infarction (MI) events; however, there was a significantly lower risk of fatal or nonfatal stroke (HR: 0.25; 95% CI, 0.08–0.75; P < .01).16 In a separate subgroup of individuals with preexisting CVD at baseline, treatment did not lead to a significant change in depression severity or CVD events between groups.16 In SMILE-II, people with MDD were randomized to sertraline, 2 different exercise regimens, or placebo for 16 weeks.17,44 When all active treatment groups (home-based exercise, supervised exercise, and sertraline) were pooled, a numeric improvement in MDD remission was observed compared with placebo, although this did not reach statistical significance (OR: 2.0; 95% CI, 0.97–4.2).44 When changes in CHD risk factors were assessed, all active treatments pooled were significantly associated with an improved composite measure of CHD risk (P = .001), reduced carotid intima-media thickness (P = .037), improved brachial artery flow–mediated dilation (P = .032), a reduction in the 10-year atherosclerotic CVD risk (P = .049), and a reduction in systolic blood pressure (SBP; P = .012) compared with placebo.17 There were no differences in serum lipid (total cholesterol [P = .159] or high-density lipoprotein [P = .894]) risk factors.17 Although this study17 did not identify CHD events per se, and provided only pooled data from both pharmacologic and exercise-based interventions, it provides evidence that MDD symptom improvement can occur alongside a reduction in CHD risk factors.
Hypertension is another risk factor for CVD, and 1 RCT included in the review (N = 311) assessed people with and without hypertension and diagnosed with depression who were randomized to treatment with duloxetine or matching placebo for 8 weeks.18 Participants receiving duloxetine reported significant improvements in HDRS (−6.49 vs −3.72; P < .001) and Geriatric Depression Scale (GDS; −4.07 vs −1.34; P < .001) scores compared with placebo after 8 weeks of treatment.18 However, among the subgroups with hypertension, there were no significant differences in the rate of treatment-emergent orthostatic hypotension between treatment groups.18 Additionally, there were no statically significant treatment-by-subgroup interactions for change from baseline to endpoint in supine and standing SBP, diastolic blood pressure (DBP), pulse pressure, or orthostatic changes in SBP and DBP for any subgroups with prerandomization hypertension.18
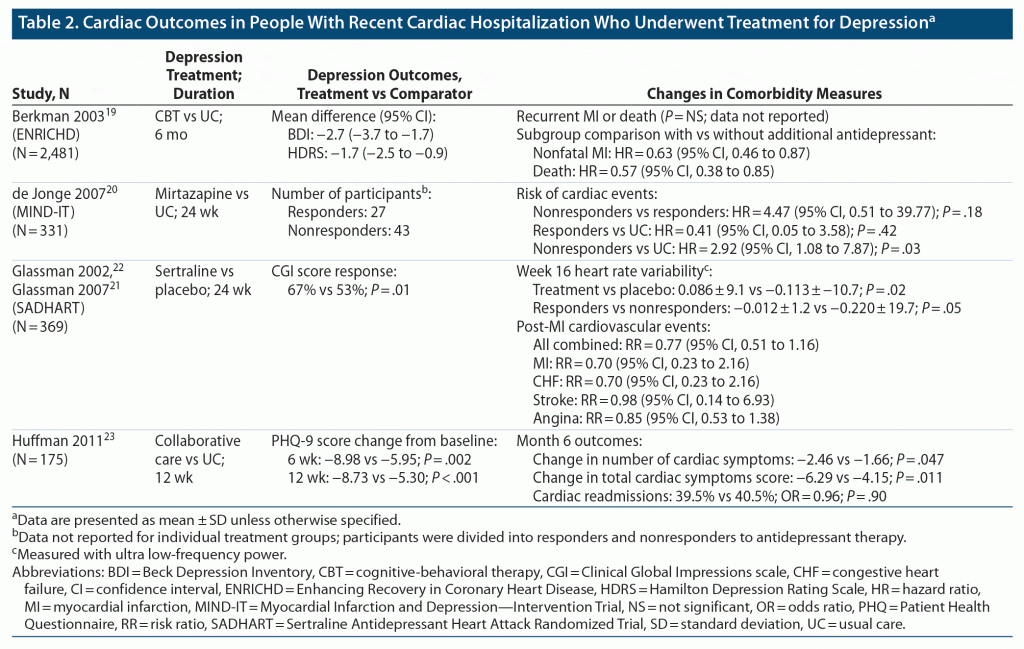
Findings from 4 RCTs (5 publications) that assessed depressive symptom improvement and the potential impact on disease severity in people with cardiac disease are summarized in Table 2.19–23 In conjunction with improvements in depressive symptoms, participants in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study19 (N = 2,481) demonstrated no significant differences in occurrence of recurrent MI or death between treatment groups (CBT vs usual care). People in the Myocardial Infarction and Depression–Intervention Trial (MIND-IT) study20 (N = 331) showed no increased risk of cardiac events for people with MDD who did not respond to mirtazapine after 24 weeks of treatment compared with responders and between mirtazapine responders and untreated controls. Individuals who participated in a collaborative care program for depression or usual care for 12 weeks (N = 175) had the same likelihood of 6-month cardiac readmissions, although the change in the number of cardiac symptoms and total score on the cardiac symptom list was significantly different between collaborative care and usual care at the 6-month follow-up.23 Furthermore, participants in the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART; N = 369) treated with sertraline demonstrated a significant improvement in change in ultra low-frequency power (an indication of functional improvement) compared with placebo.21,22 It is also notable that when study participants with improved mood, regardless of treatment group, were compared with those who did not have improved mood, there was a borderline significant difference in change in low-frequency power in SADHART (P = .05).21 However, not all findings from SADHART were different between groups as, similar to the observations from ENRICHD, there were no significant differences between groups treated with sertraline or placebo for post-MI cardiovascular events combined and separately for MI, CHF, stroke, or angina.21
One small RCT (N = 31) reported data for the impact of depression treatment on functional status in people recently hospitalized with stroke.24 This study showed borderline significant improvements in Montgomery-Asberg Depression Rating Scale scores in participants randomized to fluoxetine compared with placebo for 45 days (mean score: 11.8 vs 18.7; P = .05).24 However, there were no significant differences in mean ± SD scores of poststroke functional recovery (Motricity Index: 48.5 vs 55.3; MMSE: 24.8 vs 26.2) or Functional Independence Measure (87.4 vs 88.7) at the 45-day time point between treatment groups.24
Metabolic and Endocrine Disorders
The review identified 5 individual RCTs and 1 meta-analysis that met inclusion criteria,25–31 all of which randomized people with diabetes and depression to receive either antidepressants (sertraline, fluoxetine, or nortriptyline) or CBT/behavioral interventions in the intervention arms and evaluated the impact of the intervention or control treatment on both depressive symptoms and diabetes severity (most frequently evaluated using different measures of glycemic control). In general, all studies demonstrated a significant improvement in depression symptoms in the intervention arm compared with the control arm, but few showed evidence of concurrent between-group changes in glycemic control (Table 3).25–31 However, the meta-analysis25 showed that among studies assessing treatment with selective serotonin reuptake inhibitors (SSRIs) specifically, the standardized mean difference in glycemic control compared with control/placebo groups was significant (−0.38; 95% CI, −0.64 to −0.12). In a study26,27 that tested the Multifaceted Diabetes and Depression Program compared with usual care in 387 Hispanic adults with type 1 or type 2 diabetes and depression, the difference in glycemic control measures was not significant between treatment groups; however, there were significant time by group interactions for other measures of diabetes symptoms, functional impairment, and pain.
Autoimmune/Gastrointestinal Disorders and Musculoskeletal/Pain Conditions
This review identified 1 study in people with multiple sclerosis32,33 and 4 studies assessing the impact of depressive symptom improvement on musculoskeletal and pain outcomes,34–37 2 of which were pooled analyses of multiple RCTs.34,36 Many of these studies showed that pain outcomes and other markers of disease severity improved alongside improvements in depressive symptoms, although results were not consistent across all analyses, and it has been shown that some antidepressant therapies used (duloxetine and quetiapine) have potential analgesic effects.45,46
In the single RCT of people with multiple sclerosis (N = 127) and depression who were randomized to 16 weeks of telephone-administered therapy (CBT or supportive emotion-focused therapy),32,33 telephone CBT led to significant improvements in HDRS score over supportive emotion-focused therapy (treatment by time effect estimate: −0.17; P = .01). CBT-treated participants also demonstrated improvements over the comparator in Guy’s Neurologic Disability Scale (treatment by time effect estimate: −0.169; P = .004) and the Fatigue Impact Scale (treatment by time effect estimate: −0.302; P = .032).32,33
A pooled analysis of 2 RCTs (N = 512) assessed pain outcomes in people with MDD who were randomized to either duloxetine or placebo for 9 weeks.34 Duloxetine is an antidepressant but also has analgesic effects; to adjust for this, this study assessed the proportion of overall pain reduction that was independent of changes in depressive symptoms.34 In this analysis, both depressive symptoms and pain severity were reduced in the duloxetine-treated group compared with the placebo-treated group. This observation was consistent for most pain scores at most weekly time points (P = .016 at week 9 for overall pain). To account for the analgesic effects of the intervention, a path analysis for overall pain showed that 50.6% of duloxetine’s total effect was independent of changes in depressive symptoms, whereas 49.4% was an indirect effect mediated through change in the HDRS total score.34 Another study35 that assessed interpersonal psychotherapy compared with enhanced treatment as usual in 62 women with chronic pelvic pain showed an improvement in HDRS score for interpersonal psychotherapy over enhanced treatment (effect estimate: 2.1533; P < .05), whereas the effect on the Multidimensional Pain Inventory score was not significantly different between treatment groups.
Individuals with fibromyalgia and depression were assessed in a pooled analysis of 4 RCTs
(N = 350) wherein participants were randomized to duloxetine or placebo for 12–28 weeks.36 In this analysis, the total effect of treatment on depressive symptoms was statistically significant (P = .037). A path analysis showed that changes from baseline in Brief Pain Inventory (BPI) mean pain severity were positively correlated with the changes in HDRS total score, indicating that 68.7% of the reduction in pain was a direct effect of duloxetine, whereas the improvement in mood accounted for 31.3% of pain improvement.36 In a separate RCT of people with fibromyalgia and depression (N = 120) who were treated with quetiapine extended-release or placebo for 8 weeks,37 adjusted mean differences for quetiapine compared with placebo were observed for HDRS scores (−3.7; 95% CI, −5.9 to −1.5; P = .001), Clinical Global Impression–Severity of Depression scores (−0.6; 95% CI, −1.0 to −0.2; P = .003), HDRS response rate (25.9%; 95% CI, 9.9 to 41.9; P = .002), and HDRS remission rate (18.0%; 95% CI, 5.8 to 30.1; P = .004). At the same time, changes in disease severity measured by adjusted mean difference in pain outcomes were demonstrated for quetiapine over placebo, including for the outcomes of BPI total (−1.6; 95% CI, −2.8 to −0.5; P = .007), BPI-Severity (−0.6; 95% CI, −1.2 to 0; P = .036), and BPI-Interference (−1.0; 95% CI, −1.7 to −0.2; P = .008).37 Furthermore, symptoms of depression (HDRS scores) were moderately correlated with measures of pain (BPI-Interference) and fibromyalgia (Fibromyalgia Impact Questionnaire).37
Infectious Diseases
The review did not identify any studies that met inclusion criteria and assessed infectious disease comorbidities.
Respiratory Disorders
This review identified 1 small RCT in 36 people with chronic obstructive pulmonary disease (COPD) and depression who were randomized to treatment with nortriptyline or placebo for a 12-week period.38 A significantly greater proportion of participants in the nortriptyline-treated group experienced a depression response (77% vs 12%, P = .0003), and the mean end-of-treatment HDRS score was significantly lower (12.6 vs 22.8, P = .01) compared with the placebo group (an improvement from baseline of 60% vs 17%).38 Disease severity was measured using several assessments of dyspnea; overall, the nortriptyline-treated group showed a significantly greater improvement in symptoms associated with breathing (differential treatment effect: 5.4, P = .04) and change in Pulmonary Functional Status Instrument (differential treatment effect: 0.71, P = .002) compared with placebo-treated participants.38
Substance Abuse
In total, 3 RCTs and 2 meta-analyses of RCTs were identified that assessed the impact of interventions for depression in people with both depression and substance use disorder.39–43 Many, but not all, studies reported improvements in substance use behavior that were concurrent with improvements in depressive symptoms.
In an RCT of people with alcohol dependence (n = 71), median change in HDRS score (−13.00 vs −6.00; P < .05) and proportion of participants achieving depression response criteria (81.8% vs 22%; P = .02) was significantly improved in desipramine-treated individuals compared with placebo over a 6-month period.40 There were also fewer alcohol use relapses in the desipramine group compared with the placebo group in the depressed subgroup, but this was not statistically significant (8.3% vs 40%; P = .14).40 In a separate RCT of 51 people with alcohol dependence and MDD, treatment with fluoxetine led to a significantly greater mean change in HDRS score compared with placebo over 12 weeks (−6.0 vs −2.0; P < .05).39 Concurrent improvements were observed in several measures of drinking behavior including cumulative drinks (70.2 vs 215.5; P < .03), cumulative number of drinking days (10.6 vs 20.3; P < .05), drinks per drinking day (2.4 vs 5.4; P < .05), cumulative number of days of heavy drinking (4.8 vs 16.0; P = .04), and number of weeks until first heavy drinking (8.0 vs 4.7; P < .02).39
A meta-analysis assessed quantity of substance use in people with alcohol, cocaine, or opioid dependence who received antidepressant therapy compared with placebo.41 In this analysis, a pooled improvement in depressive symptoms was noted for participants with alcohol dependence who were treated with antidepressants other than SSRIs (OR: 4.15; 95% CI, 1.35–12.75).41 These same studies showed no reduced alcohol use among non–SSRI-treated individuals compared with those treated with placebo (OR: 1.99; 95% CI, 0.78–5.08).41 Studies included in the meta-analysis that assessed either cocaine or opioid dependence showed no significant change in depressive symptoms following antidepressant treatment compared with placebo and therefore were not relevant for this analysis. It is notable, however, that treatment with antidepressants did lead to a significant improvement (ie, reduction) in illicit opioid use compared with placebo in people with depression (OR: 3.65; 95% CI, 1.10–12.16), despite no significant improvement in depressive symptoms.41
Another RCT assessed 137 people with depression who were newly admitted to a methadone-based treatment program and randomized to imipramine hydrochloride or placebo.43 Following 12 weeks of therapy, both the depression response (67% vs 26%; P = .001) and HDRS score (8.0 vs 13.6; P < .001) were significantly improved in the imipramine group compared with the placebo group.43 Furthermore, concurrent improvements were observed in some measures of substance abuse in participants treated with imipramine hydrochloride compared with placebo, including days per week craving a substance (2.7 vs 4.5; P = .003) and intensity of craving (1.6 vs 2.3; P = .006).43 Although the proportion of study participants with urine-confirmed abstinence also showed a numeric improvement following treatment with imipramine compared with placebo, it was not statistically significant (14% vs 2%; P = .11).43
A separate meta-analysis of 15 RCTs (N = 848) assessed the impact of antidepressant treatment in people with depression and substance use disorder (both drugs and alcohol).42 Studies were stratified based on effect of treatment on depressive symptoms. Among studies with a depression effect size > 0.50 (ie, stronger improvement in depressive symptoms for intervention vs placebo), the pooled effect size on the quantity of substance abuse was significantly improved (0.56; 95% CI, 0.33–0.79), whereas no significant difference was observed among studies with a depression effect size < 0.50.42
Quality Assessment
According to the risk of bias assessment across RCTs, most studies had a low or unclear risk of selection bias and attrition bias. Nearly all studies were determined to have an unclear risk of detection bias, which was primarily because of a lack of clarity regarding whether investigators were blinded to other important confounding factors. In addition, studies were evenly split between low and high risk of performance bias. Reasons for high risk gradings were typically a lack of blinding to treatment among participants and/or those administering care, although this was often not possible for behavioral intervention studies. Further details of the quality assessment for individual studies are reported in Supplementary Tables 3–5.
DISCUSSION
Mental health, including both its improvement and decline, is intricately linked with changes in overall health. For people with certain comorbid disorders and MDD, effective treatment of depression occurred alongside a significant improvement in the severity of their comorbid disease compared to less effective treatment. This trend was demonstrated for people with Parkinson’s disease, multiple sclerosis, diabetes pain and symptom severity (but not glycemic control outcomes), COPD, and certain pain conditions. Effective treatment of depression was also associated with a decrease in the risk of CVD events in otherwise healthy individuals and the risk of cardiac symptoms (but not events) in people with prior cardiac hospitalizations. Findings in people with substance use disorders were somewhat mixed, although several included studies reported decreases in alcohol and substance use alongside improvements in depression symptoms. There were no observed differences in disease severity/course for cancer, Alzheimer’s disease, and poststroke recovery in conjunction with improvements in depressive symptoms.
Constraints associated with study designs complicated the identification of clinically meaningful findings. For example, although the included studies demonstrated statistically significant improvements in depression symptoms with treatment compared with controls, the effect sizes observed following depression treatment were often relatively small, which could impact the ability to observe meaningful effects on associated comorbidities. Furthermore, the included studies had heterogeneous designs, with considerably different methods, populations, follow-up durations, and analyses. It should also be noted that the time frames used in many studies (eg, 12 weeks) may not have been a sufficient length to capture the long-term positive impact of these interventions on comorbid outcomes. Some studies12,20,21,29,30 conducted additional analyses in depression treatment responders compared with nonresponders, regardless of the treatment received. In 2 of 5 studies,21,29 these analyses led to statistically significant (or borderline significant) changes in comorbidity outcomes that had not been observed between treatment groups.
Similar findings to those of this review have been reported previously. In a literature review of the association between depression and pain, most of the studies that assessed whether antidepressant treatment was effective for pain symptoms (22 in total) reported that participants experienced improvements in both pain and depression symptoms.47 It should be acknowledged, however, that antidepressants may be directly impacting pain pathways in the brain rather than modulating pain through their effects on depressive symptoms.48 The inability to distinguish causality is a limitation of this systematic review and is discussed in further detail below. A separate review that examined the relationship between depression and CVD concluded that, although the association between depression and the incidence or worsening of CVD was well established, the question of whether effective depression treatment could lead to subsequent improvement in CVD did not yet have adequate supporting evidence.49
This review contains several limitations. Observed results from the included studies demonstrated a correlation between improvements in depressive symptoms and changes in comorbidity outcomes in certain diseases, but this must be interpreted with caution. The mechanism of action of certain antidepressants (including analgesic effects of duloxetine and quetiapine, which were assessed in patients with chronic pain) may have a direct impact on comorbidities, which inherently cannot be controlled for. It therefore remains possible that the comorbidity could be directly improved by the treatment. Alternatively, positive changes resulting from MDD improvement could be weakened by direct negative adverse effects of therapy. One example of such negative treatment impacts is the observation that SSRI therapy is associated with patient-reported weight gain,50 thus improvements in metabolic and endocrine outcomes that are directly impacted by depression symptoms could be offset by the increased risk of obesity. In a handful of included studies, analyses were conducted to account for the potential direct impact of the antidepressant agent on comorbidity outcomes. Additional studies designed to adjust for appropriate covariates could help to further uncover the causal association between depression improvement and downstream effects on comorbid diseases.
The findings of this review further underscore the need to continue integrating general and mental health care, for example through routine screening for mental health disorders in a primary care environment. Such actions could lead to more rapid identification and coordinated, efficient management of both physical and psychiatric diseases. Findings from programs that piloted an integrated care model have shown that they lead to improvements in participants’ access to health care, satisfaction with care, and health outcomes as well as a greater willingness of their providers to address mental health issues within the primary care setting.51,52
In conclusion, effective treatment of MDD may lead to an improvement in the incidence and severity of certain serious comorbidities. These results highlight the importance of appropriate and timely treatment of MDD, particularly among those suffering from comorbid conditions.
Submitted: May 31, 2022; accepted August 3, 2022.
Published online: January 3, 2023.
Relevant financial relationships: Ms Suthoff is a current employee and Ms Arnaud and Dr Werneburg are former employees of Sage Therapeutics, Inc and own stock/stock options. Drs Reinhart and Aleksanderek are current or former employees of Tantalus Medical Communications, Ltd, which received financial support from Sage Therapeutics, Inc and Biogen, Inc for research and medical writing support. During the peer review process, both Sage Therapeutics, Inc and their collaboration partner Biogen, Inc had the opportunity to review and comment on this manuscript; however, the authors had full editorial control of the manuscript and provided final approval on all content. Ms Fulwider has received consulting fees from Sage Therapeutics, Inc. Drs Brister and Duckworth and Ms Foxworth have nothing to disclose.
Funding/support: This work was supported by Sage Therapeutics, Inc and Biogen, Inc.
Role of the sponsor: Employees of Sage Therapeutics, Inc contributed to the protocol design and manuscript drafting and review but had no role in the screening and study selection for inclusion in the systematic review.
Previous presentation: This work has not been presented previously.
Acknowledgments: The authors thank Katie Stenson, MSc, a former employee of Sage Therapeutics, Inc, for contributions to the design and interpretation of the systematic review and Shawn Eapen, MSc, and Sara Watchko, MPH, both affiliates of Tantalus Medical Communications (Victoria, British Columbia, Canada), for assistance with the systematic review. Mr Eapen and Mss Stenson and Watchko have no conflicts of interest to declare. The authors thank Tantalus Medical Communications for providing research and medical writing support, which was funded by Sage Therapeutics, Inc and Biogen, Inc in accordance with Good Publication Practice (GPP3) guidelines.
Supplementary material: See accompanying pages.
Clinical Points
- Although there are many well-studied comorbidities among people with depression, the impact of treating depression on the severity or risk of serious comorbidities has not been widely tested.
- Effective treatment of depression may have a positive impact on the severity of certain comorbidities and thus overall patient health.
- Integration of general and mental health care through routine screening for mental health disorders should be considered in a primary care environment along with appropriate and timely treatment of MDD.
References (52)

- Bhattacharya R, Shen C, Sambamoorthi U. Excess risk of chronic physical conditions associated with depression and anxiety. BMC Psychiatry. 2014;14(1):10. PubMed CrossRef
- Kang HJ, Kim SY, Bae KY, et al. Comorbidity of depression with physical disorders: research and clinical implications. Chonnam Med J. 2015;51(1):8–18. PubMed CrossRef
- Arnaud AM, Brister TS, Duckworth K, et al. Impact of major depressive disorder on comorbidities: a systematic literature review. J Clin Psychiatry. 2022;83(6):21r14328. PubMed CrossRef
- Blue Cross Blue Shield Association. Major depression: the impact on overall health. Published 2018. Accessed February 5, 2020. https://www.bcbs.com/sites/default/files/file-attachments/health-of-america-report/HoA_Major_Depression_Report.pdf
- Armbrecht E, Shah A, Schepman P, et al. Economic and humanistic burden associated with noncommunicable diseases among adults with depression and anxiety in the United States. J Med Econ. 2020;23(9):1032–1042. PubMed CrossRef
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. PubMed CrossRef
- National Institute for Health and Care Excellence. The social care guidance manual Appendix B Methodology checklist: systematic reviews and meta-analyses. Published 2013. Accessed February 5, 2020. https://www.nice.org.uk/process/pmg10/chapter/appendix-b-methodology-checklist-systematic-reviews-and-meta-analyses
- National Institute for Health and Care Excellence. The social care guidance manual Appendix C Methodology checklist: randomized controlled trials. Published 2012. Accessed February 5, 2020. https://www.nice.org.uk/process/pmg6/resources/the-guidelines-manual-appendices-bi-2549703709/chapter/appendix-c-methodology-checklist-randomised-controlled-trials
- Walker J, Hansen CH, Martin P, et al; SMaRT (Symptom Management Research Trials) Oncology-3 Team. Integrated collaborative care for major depression comorbid with a poor prognosis cancer (SMaRT Oncology-3): a multicentre randomised controlled trial in patients with lung cancer. Lancet Oncol. 2014;15(10):1168–1176. PubMed CrossRef
- Mulick A, Walker J, Puntis S, et al. Does depression treatment improve the survival of depressed patients with cancer? A long-term follow-up of participants in the SMaRT Oncology-2 and 3 trials. Lancet Psychiatry. 2018;5(4):321–326. PubMed CrossRef
- Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry. 2003;60(7):737–746. PubMed CrossRef
- Munro CA, Brandt J, Sheppard JM, et al. Cognitive response to pharmacological treatment for depression in Alzheimer disease: secondary outcomes from the depression in Alzheimer’s disease study (DIADS). Am J Geriatr Psychiatry. 2004;12(5):491–498. PubMed CrossRef
- Thompson S, Herrmann N, Rapoport MJ, et al. Efficacy and safety of antidepressants for treatment of depression in Alzheimer’s disease: a metaanalysis. Can J Psychiatry. 2007;52(4):248–255. PubMed CrossRef
- Dobkin RD, Menza M, Allen LA, et al. Cognitive-behavioral therapy for depression in Parkinson’s disease: a randomized, controlled trial. Am J Psychiatry. 2011;168(10):1066–1074. PubMed CrossRef
- Dobkin RD, Tröster AI, Rubino JT, et al. Neuropsychological outcomes after psychosocial intervention for depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2014;26(1):57–63. PubMed CrossRef
- Stewart JC, Perkins AJ, Callahan CM. Effect of collaborative care for depression on risk of cardiovascular events: data from the IMPACT randomized controlled trial. Psychosom Med. 2014;76(1):29–37. PubMed CrossRef
- Sherwood A, Blumenthal JA, Smith PJ, et al. Effects of exercise and sertraline on measures of coronary heart disease risk in patients with major depression: results from the SMILE-II randomized clinical trial. Psychosom Med. 2016;78(5):602–609. PubMed CrossRef
- Raskin J, Wiltse CG, Dinkel JJ, et al. Safety and tolerability of duloxetine at 60 mg once daily in elderly patients with major depressive disorder. J Clin Psychopharmacol. 2008;28(1):32–38. PubMed CrossRef
- Berkman LF, Blumenthal J, Burg M, et al; Enhancing Recovery in Coronary Heart Disease Patients Investigators (ENRICHD). Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–3116. PubMed CrossRef
- de Jonge P, Honig A, van Melle JP, et al; MIND-IT Investigators. Nonresponse to treatment for depression following myocardial infarction: association with subsequent cardiac events. Am J Psychiatry. 2007;164(9):1371–1378. PubMed CrossRef
- Glassman AH, Bigger JT, Gaffney M, et al. Heart rate variability in acute coronary syndrome patients with major depression: influence of sertraline and mood improvement. Arch Gen Psychiatry. 2007;64(9):1025–1031. PubMed CrossRef
- Glassman AH, O’Connor CM, Califf RM, et al; Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–709. PubMed CrossRef
- Huffman JC, Mastromauro CA, Sowden G, et al. Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;4(2):198–205. PubMed CrossRef
- Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829–1832. PubMed CrossRef
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus: an abridged Cochrane review. Diabet Med. 2014;31(7):773–786. PubMed CrossRef
- Ell K, Katon W, Xie B, et al. Collaborative care management of major depression among low-income, predominantly Hispanic subjects with diabetes: a randomized controlled trial. Diabetes Care. 2010;33(4):706–713. PubMed CrossRef
- Ell K, Katon W, Xie B, et al. One-year postcollaborative depression care trial outcomes among predominantly Hispanic diabetes safety net patients. Gen Hosp Psychiatry. 2011;33(5):436–442. PubMed CrossRef
- Lustman PJ, Griffith LS, Clouse RE, et al. Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom Med. 1997;59(3):241–250. PubMed CrossRef
- Lustman PJ, Griffith LS, Freedland KE, et al. Cognitive behavior therapy for depression in type 2 diabetes mellitus: a randomized, controlled trial. Ann Intern Med. 1998;129(8):613–621. PubMed CrossRef
- Lustman PJ, Freedland KE, Griffith LS, et al. Fluoxetine for depression in diabetes: a randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23(5):618–623. PubMed CrossRef
- Petrak F, Herpertz S, Albus C, et al. Cognitive behavioral therapy versus sertraline in patients with depression and poorly controlled diabetes: the Diabetes and Depression (DAD) study: a randomized controlled multicenter trial. Diabetes Care. 2015;38(5):767–775. PubMed CrossRef
- Kinsinger SW, Lattie E, Mohr DC. Relationship between depression, fatigue, subjective cognitive impairment, and objective neuropsychological functioning in patients with multiple sclerosis. Neuropsychology. 2010;24(5):573–580. PubMed CrossRef
- Mohr DC, Hart S, Vella L. Reduction in disability in a randomized controlled trial of telephone-administered cognitive-behavioral therapy. Health Psychol. 2007;26(5):554–563. PubMed CrossRef
- Fava M, Mallinckrodt CH, Detke MJ, et al. The effect of duloxetine on painful physical symptoms in depressed patients: do improvements in these symptoms result in higher remission rates? J Clin Psychiatry. 2004;65(4):521–530. PubMed CrossRef
- Poleshuck EL, Gamble SA, Bellenger K, et al. Randomized controlled trial of interpersonal psychotherapy versus enhanced treatment as usual for women with co-occurring depression and pelvic pain. J Psychosom Res. 2014;77(4):264–272. PubMed CrossRef
- Marangell LB, Clauw DJ, Choy E, et al. Comparative pain and mood effects in patients with comorbid fibromyalgia and major depressive disorder: secondary analyses of four pooled randomized controlled trials of duloxetine. Pain. 2011;152(1):31–37. PubMed CrossRef
- McIntyre A, Paisley D, Kouassi E, et al. Quetiapine fumarate extended-release for the treatment of major depression with comorbid fibromyalgia syndrome: a double-blind, randomized, placebo-controlled study. Arthritis Rheumatol. 2014;66(2):451–461. PubMed CrossRef
- Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190–201. PubMed CrossRef
- Cornelius JR, Salloum IM, Ehler JG, et al. Fluoxetine in depressed alcoholics: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1997;54(8):700–705. PubMed CrossRef
- Mason BJ, Kocsis JH, Ritvo EC, et al. A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. JAMA. 1996;275(10):761–767. PubMed CrossRef
- Torrens M, Fonseca F, Mateu G, et al. Efficacy of antidepressants in substance use disorders with and without comorbid depression: a systematic review and meta-analysis. Drug Alcohol Depend. 2005;78(1):1–22. PubMed CrossRef
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291(15):1887–1896. PubMed CrossRef
- Nunes EV, Quitkin FM, Donovan SJ, et al. Imipramine treatment of opiate-dependent patients with depressive disorders: a placebo-controlled trial. Arch Gen Psychiatry. 1998;55(2):153–160. PubMed CrossRef
- Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. PubMed CrossRef
- Enomoto H, Fujikoshi S, Funai J, et al. Assessment of direct analgesic effect of duloxetine for chronic low back pain: post hoc path analysis of double-blind, placebo-controlled studies. J Pain Res. 2017;10:1357–1368. PubMed CrossRef
- Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med (Korean Assoc Intern Med). 2017;32(6):1069–1074. PubMed CrossRef
- Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. PubMed CrossRef
- Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26(1):30–36. PubMed
- Glassman AH. Depression and cardiovascular comorbidity. Dialogues Clin Neurosci. 2007;9(1):9–17. PubMed CrossRef
- Cascade E, Kalali AH, Kennedy SH. Real-world data on SSRI antidepressant side effects. Psychiatry (Edgmont). 2009;6(2):16–18. PubMed
- Brawer PA, Martielli R, Pye PL, et al. St. Louis Initiative for Integrated Care Excellence (SLI(2)CE): integrated-collaborative care on a large scale model. Fam Syst Health. 2010;28(2):175–187. PubMed CrossRef
- Reiss-Brennan B. Mental health integration: normalizing team care. J Prim Care Community Health. 2014;5(1):55–60. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top