ABSTRACT
Objective: To assess the safety and tolerability of folinic acid as an augmentation agent among geriatric inpatients with depression and to determine whether this augmentation was associated with decreased days of clinically needed hospitalization
Methods: A retrospective chart review was conducted of the medical records of patients > 60 years of age discharged from a geriatric psychiatry unit between June 1, 2014, and February 1, 2016. Two groups were compared: those with depression who received folinic acid (leucovorin) supplementation (n = 35) and those with depression who did not receive leucovorin (n = 80). The primary outcome measure was number of clinically needed days of hospitalization.
Results: The mean ± SD number of days (logged) of clinically needed hospitalization in the leucovorin group was 2.0 ± 0.7 compared with 2.4 ± 0.6 in the nonintervention group. Unpaired t group analysis yielded the following: t115 = 3.47, P < .0001 (2-tailed).
Conclusions: The patients who received leucovorin had a significantly reduced requirement of days of hospitalization, which translates to reduced cumulative cost of treatment during hospitalization. Further studies, including randomized controlled trials, are recommended to explore this treatment option.
Prim Care Companion CNS Disord 2021;23(2):20m02767
To cite: Saxena PP, Kyomen H. Leucovorin as an antidepressant adjunct in elderly inpatients with treatment-resistant depression. Prim Care Companion CNS Disord. 2021;23(2):20m02767.
To share: https://doi.org/10.4088/PCC.20m02767
© Copyright 2021 Physicians Postgraduate Press, Inc.
aBrockton Neighborhood Health Center, Brockton, Massachusetts
bBoston University School of Medicine, Boston, Massachusetts
cTufts University School of Medicine, Boston, Massachusetts
dHarvard Medical School, Boston, Massachusetts
*Corresponding author: Parnika Prashasti Saxena, MD, Brockton Neighborhood Health Center, 63 Main St, Brockton, MA 02301 ([email protected]).
Depression is a rising cause of morbidity in the world, and it is estimated that 7 million Americans above the age of 65 years are affected.1 Late-life depression can be challenging to treat and can be associated with a poor prognosis.2,3 Among those who seek treatment, there is a significant number who have limited response to standard antidepressant therapy and might suffer from treatment-resistant depression (TRD).4 The prevalence of treatment-resistant depression was demonstrated in the Sequenced Treatment Alternative to Relieve Depression (STAR*D) trial wherein it was concluded that no more than one-third of patients treated with standard antidepressants achieved remission.5 Thus, there is great interest in discovering newer modalities of depression treatment including adjunctive treatments. In the elderly, tolerance and a lower risk of adverse effects resulting from treatment is of the utmost importance.
It is hypothesized that folate levels (in the serum and cerebrospinal fluid [CSF]) influence depressive illness through multiple etiologies.6 Folate is a B vitamin that is not synthesized de novo and requires supplementation by dietary means. Several studies since the 1960s have shown that a significant number of patients with depression have reduced folate levels.6–12 This is pertinent in older adults, as there is a high incidence of folate deficiency in this age demographic13 as well as evidence that depression in older adults is associated with low folate levels.14 Furthermore, patients with red blood cell folate deficiency have reduced CSF 5HIAA (5 hydroxyindole acetic acid) levels, which is a serotonin metabolite.15 This is significant, as reduced CSF 5HIAA levels are associated with aggressive and suicidal behaviors.16,17
Folate is also associated with the amino acid derivative of methionine: homocysteine. High levels of homocysteine are associated with low folate levels and CSF SAMe (S-adenosyl-L-methionine).15 SAMe is involved in the production of neurotransmitters such as serotonin, epinephrine, and dopamine by acting as a methyl donor.6 Another mechanism by which folate might be involved in depression is by impacting the regeneration of BH4 (tetrahydrobiopterin).18 BH4 acts as a rate-limiting enzyme cofactor to the hydroxylase enzymes that metabolize tryptophan to 5-hydroxytryptophan, phenylalanine to tyrosine, and tyrosine to dopa.6
Therefore, folate deficiency and treatment of the same are implicated in depressive illnesses,19 with some studies20,21 reporting lower rates of response to standard antidepressant treatment in individuals with low serum folate. Two studies by Coppen and colleagues,22,23 in which folic acid was added to patient regimens of antidepressants, showed significant improvement in depressive symptoms.
Unfortunately, dietary folate only has absorption of 50% compared to synthetic folic acid, which has an absorption rate of 85%–90%.6 However interventional studies done after implementation of the folic acid fortification program in the United States in 199824 demonstrated that depressed patients with normal to low normal serum folate levels did not respond to folate supplementation, suggesting that these patients either require a higher level of folate or that their CSF folate levels were not high enough. One of the hypothesized reasons might be the presence of genetic polymorphisms of methylenetetrahydrofolate reductase, which is an enzyme involved in folate metabolism and required for regulation of 5-methyltetrahydrofolate, the active folate metabolite.6 Its genetic polymorphisms can alter folate metabolism; for example, the C677 variant (which is not uncommon in the United States) is associated with lower levels of red blood cell folate, serum folate, and vitamin B12. Reviews9 have also concluded that this polymorphism is associated with increased risk of depression.
Therefore, supplementation has been the area of interest, preferably with compounds that cross the blood-brain barrier to enter the central nervous system. This would also suggest that plasma folate levels might not be an accurate reflection of CSF folate levels. Folate supplements like folinic acid and methyltetrahydrofolate are well tolerated and have the advantage of not requiring dihydrofolate reductase for conversion into an active form of folate. For example, folinic acid is converted to l-methylfolate by methylenetetrahydrofolate reductase, which enters the blood-brain barrier.6 Similarly, methyltetrahydrofolate also actively crosses the blood-brain barrier and has been studied as monotherapy and adjunctive treatment for depression. Most studies using methyltetrahydrofolate as supplementation for depressed individuals have shown positive results25–30 except for one.31
Unfortunately, the administration of methylfolate or methyltetrahydrofolate can be cost prohibitive. Folinic acid or leucovorin also crosses the blood-brain barrier and is relatively more cost effective. It has, however, not been studied to the extent that methylfolate has been. Currently, it is US Food and Drug Administration approved to be given adjunctively with methotrexate therapy in neoplasms at high doses; however, for depressive disorders, much lower doses have been tried.32 A study by Alpert et al32 assessed the effect of leucovorin as an adjunctive antidepressant in adults. Adults with a mean ± SD age of 45.2 ± 11.0 years with treatment-refractory depression (with either a selective serotonin reuptake inhibitor [SSRI] or venlafaxine) were treated with leucovorin for 8 weeks (dosing: 15 mg/d for 2 weeks, followed by 30 mg/d for 6 weeks). All completers showed a significant response in depression rating scales, with 31% demonstrating a response and 19% entering remission.32
Given these data, we decided to study the impact of folinic acid supplementation in patients on our acute inpatient geropsychiatric unit by studying days of clinically needed hospitalization. These data are especially important not just from a clinical standpoint but also from a fiscal perspective. In current clinical practice, the cost of health care is an important patient care concern. In 2012, the average cost of a psychiatric inpatient hospitalization for an illness (not including a comorbid substance use disorder) was approximately $807.23 per day according to the Agency for Healthcare Research and Quality.33 However, this number is averaged for all ages, and, unfortunately, information for the elderly population is unavailable, although it is known that their length of stay is usually longer and they are at a higher risk for readmission. Although most of the studies described here were conducted in an outpatient setting, ours was conducted with inpatients. A study by Bell and colleagues34 on a geropsychiatric inpatient unit revealed that low folate levels correlated negatively with length of hospitalization.
METHODS
A retrospective chart review was conducted of the medical records of patients > 60 years of age discharged from the St. Elizabeth’s Medical Center Geriatric Psychiatry Unit between June 1, 2014, and February 1, 2016. The study was approved by the St. Elizabeth’s Medical Center Institutional Review Board.
The charts of patients diagnosed with either major depressive disorder or bipolar disorder (depressive phase) were selected for detailed review. Patients with a diagnosis of schizoaffective disorder or substance-induced mood disorder were excluded since folate supplementation has only been studied in primary depressive disorders. Patients with comorbid alcohol abuse were excluded given that alcoholism by itself can cause folate deficiency and subsequently contribute to depression. Those patients who had received SSRI, serotonin-norepinephrine reuptake inhibitor (SNRI), or noradrenergic and specific serotonergic antidepressant (NaSSA) treatment augmented with leucovorin were compared with a similar group who had not received leucovorin. Of note, there was 1 primary attending physician on this unit (H.K.), and leucovorin was added to the patients’ regimens based on patient choice as well as either a limited response to the existing regimen or a potential risk of adverse effects with an increase in dose compared to therapeutic benefit. None of the patients refused this augmentation, and in the primary attending physician’s experience, the earliest response to treatment was noted in 3 to 4 days. The dose of 25 mg was decided on for 2 reasons: (1) that it was the lowest dose available in the hospital pharmacy and would be titrated appropriately and (2) the only other article that discussed leucovorin augmentation had trialed doses between 15 and 30 mg/day.32 The patients were usually deemed ready for safe discharge to the outpatient clinician when their functionality had adequately improved.
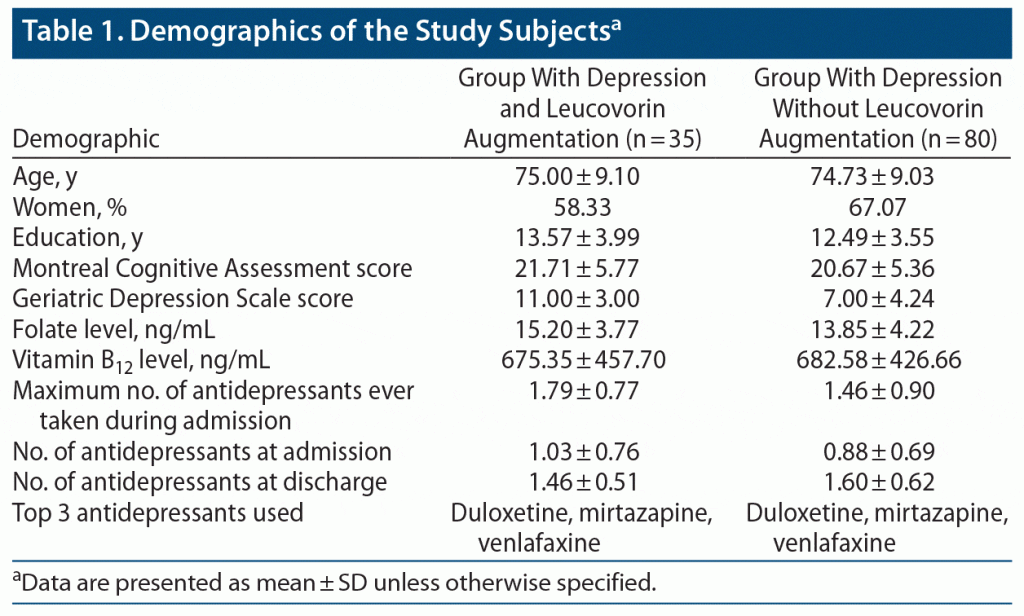
Factors compared included age, sex, education level, Montreal Cognitive Assessment (MoCA)35 score, Geriatric Depression Scale (GDS)36 score, admission serum folate level, admission serum vitamin B12 level, maximum number of antidepressants ever taken during the admission, number of antidepressants at admission or discharge, top 3 antidepressants used, leucovorin-associated adverse effects, and the number of days of hospitalization. Since this was a retrospective review, 2 variables (GDS and MoCA scores) were not available for all patients and thus were excluded from review.
The deidentified data were managed using REDCap. T tests and χ2 tests were used to compare group characteristics. Pearson correlation was used to test for an association between length of stay and drug dosage, and scatterplots were created for visual assessment. Linear regression was utilized to evaluate the associations between length of stay and vitamin D, vitamin B12, and leucovorin. As length of stay was highly skewed, the logged value was used in all analyses. Data were analyzed using Microsoft Excel and SAS 9.2. Statisticians from the Tufts School of Medicine, Boston, Massachusetts helped to design this study and completed the statistical analysis.
The hypothesis of this pilot study was 2-fold: the first was to assess the safety and tolerability of leucovorin as an augmentation agent and the second was to assess whether this augmentation was associated with decreased days of clinically needed hospitalization, which was considered to be the most appropriate clinical indicator of improvement.
RESULTS
We reviewed 539 charts. Thirty-five patients with depression who had received SSRI/SNRI/NaSSA treatment augmented with leucovorin calcium 25 mg daily (leucovorin group) were identified. These patients were compared with 80 patients with depression who had not received leucovorin (non-leucovorin group).
No significant differences were found between the leucovorin and non-leucovorin groups in age, sex distribution, education level, MoCA score, GDS score, serum folate level, serum vitamin B12 level, maximum number of antidepressants ever taken during the admission, number of antidepressants at admission or discharge, and top 3 antidepressants used (Table 1). The mean ± SD age of patients in the leucovorin group was 75.0 ± 9.1, whereas in the control group it was 74.73 ± 9.03. No differences were found in education levels assessed by total number of years of education. The admission serum folate level in the leucovorin group was 15.20 ± 3.77 ng/mL compared to 13.85 ± 4.22 ng/mL in the control group. Thus, both groups were normofolatemic. Also, the top 3 antidepressants used in both groups were the same: duloxetine, venlafaxine, and mirtazapine. At discharge, all patients had subjective improvement in mood and cessation of suicidality (in patients in whom suicidal ideation was a presenting symptom) and were stable enough to function in a nonpsychiatric inpatient facility. The leucovorin group had no adverse effects, while one person in the control group had lightheadedness and another had weakness.
With regard to efficacy of treatment, the mean number of days (logged) of clinically needed hospitalization in the leucovorin group was 2.0 ± 0.7 compared to 2.4 ± 0.6 days in the non-leucovorin group. The difference in the mean number of days (logged) of clinically needed hospitalization between the 2 groups was 0.42 days (logged), which is equivalent to 1.5 days on the original scale. Unpaired t test results comparing the leucovorin group’s number of days of clinically needed hospitalization with the non-leucovorin group’s number of days of clinically needed hospitalization yielded t115 = 3.47, P < .0001 (2-tailed), which is considered statistically significant.
We evaluated whether serum vitamin B12 level or thyroid-stimulating hormone level were significantly associated with the logged value of length of stay. No significant associations were found. Other variables such as comorbid benzodiazepine use, GDS score, and folate levels were available for too few individuals to allow for an analysis. We also analyzed the association between dose and logged length of stay separately for 3 antidepressants (most used) to analyze the role of the antidepressants in the outcome, and scatterplot diagrams were used for the same. Five patients with extreme outlier data points were removed from this analysis. There were no significant trends noted with doses of duloxetine (n = 37, correlation = 0.07, P = .68) or venlafaxine (n = 12, correlation = 0.11, P = .73). Mirtazapine appeared to show a positive relationship in that higher doses of the medication were associated with longer length of stay, but it was not statistically significant (n = 47, correlation = 0.17, P = .24). Due to the small sample size, care should be taken when interpreting the scatterplots (Figure 1).
DISCUSSION
This retrospective chart review of 115 eligible patients tested the hypotheses that leucovorin could be safely administered to the elderly population and that it is an effective intervention for patients with depression.
Our study did show that leucovorin was well tolerated with no incidence of adverse effects in the geriatric psychiatry inpatients. This finding is significant given that most of these patients had various medical comorbidities and that drug-drug interactions and worsening of preexisting medical conditions are a concern in the elderly population whenever a new intervention is introduced. This finding is similar to that of Alpert et al32 who found leucovorin to be well tolerated, and although a few patients had adverse effects such as headaches and nausea, the dropouts were due to inefficacy.
As far as efficacy is concerned, the number of clinically needed days of hospitalization was significantly less in patients receiving leucovorin compared to the group who did not receive the intervention. This result is further strengthened by the finding that the demographics and GDS and MoCA scores were not significantly different between the 2 groups. Fiscally, this result is significant as well. Given that inpatient hospitalizations cost approximately $807.23 per day (as per the latest 2012 Healthcare Cost Utilization Project data33), a decrease in 1.5 days could potentially save $1,211 per visit. No studies have been performed, to our knowledge, with folinic acid in an inpatient setting; however, as mentioned previously, Bell et al34 demonstrated that geropsychiatric patients with lower serum folate levels had longer hospitalizations. Alpert et al,32 who conducted a study with folinic acid in depressed outpatients, did notice a modest significant response to treatment (31%), and 19% exhibited remission. Thus, our results concur with the demonstration of treatment efficacy with folinic acid.
Unfortunately, since this was a retrospective review, we were unable to incorporate scores such as GDS for all patients—both preadmission and discharge scores. It is important to note that discharge criteria for inpatients focus more on stability, cessation of suicidality, and decrease in symptom severity rather than remission of illness. Therefore, clinically needed days of hospitalization is a much more appropriate measure of response.
The finding that both groups were normofolatemic has been seen in other studies including those by Guaraldi et al,25 Passeri et al,30 and Alpert et al.32 This finding has raised the thought that the clinical focus should be folate supplementation and not replacement. This perspective has become increasingly significant given that most of the studies that showed correlation between depression and low serum folate levels occurred prior to the fortification of food in the United States, despite which depression continues as an illness. Fortification does not include forms of folate that actively cross the blood-brain barrier, and this might explain the persistence of depressive disorders.
This study is limited by the small sample size and needs to be replicated in larger studies, which allow for a placebo-controlled trial. Other limitations include nonavailability of data given the study’s retrospective nature especially for cognition, use of nonstandardized measures (including lack of standardized rating scales), and the presence of a single attending physician on the inpatient unit, which adds to bias with respect to treatment decisions. However, this is the first study using leucovorin in an inpatient setting, and the significance of the results, especially in such a setting wherein patients usually have increased severity of illness, merits further studies for this intervention.
Submitted: July 26, 2020; accepted November 13, 2020.
Published online: April 22, 2021.
Potential conflicts of interest: None.
Funding/support: Statistical support was provided by the National Center for Advancing Translational Sciences, National Institutes of Health award no. UL1TR002544.
Role of the sponsor: The National Center for Advancing Translational Sciences, National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; or preparation, review, or approval of the manuscript.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments: The authors thank Lori Lyn Price, MAS, and Tara Fleckner, MPH, statisticians from the Tufts Clinical and Translational Science Institute, who helped in statistical analysis of the data. Mss Price and Fleckner report no conflicts of interest related to the subject of this article.
Clinical Points
- Treatment-resistant depression, especially in older adults, is challenging to treat.
- A lengthier duration of psychiatric hospitalization can worsen outcomes.
- Leucovorin is an effective and well-tolerated augmentation treatment for depression.
References (36)

- Steinman LE, Frederick JT, Prohaska T, et alLate Life Depression Special Interest Project (SIP) Panelists. Recommendations for treating depression in community-based older adults. Am J Prev Med. 2007;33(3):175–181. PubMed CrossRef
- Licht-Strunk E, van der Windt DA, van Marwijk HW, et al. The prognosis of depression in older patients in general practice and the community. s systematic review. Fam Pract. 2007;24(2):168–180. PubMed CrossRef
- Knöchel C, Alves G, Friedrichs B, et al. Treatment-resistant late-life depression: challenges and perspectives. Curr Neuropharmacol. 2015;13(5):577–591. PubMed CrossRef
- Nierenberg AA, Amsterdam JD. Treatment-resistant depression: definition and treatment approaches. J Clin Psychiatry. 1990;51(suppl):39–47, discussion 48–50. PubMed
- Fava M, Rush AJ, Trivedi MH, et al. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatr Clin North Am. 2003;26(2):457–494, x. PubMed CrossRef
- Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008;13(3):216–226. PubMed
- Shorvon SD, Carney MWP, Chanarin I, et al. The neuropsychiatry of megaloblastic anaemia. BMJ. 1980;281(6247):1036–1038. PubMed CrossRef
- Reynolds EH, Preece JM, Bailey J, et al. Folate deficiency in depressive illness. Br J Psychiatry. 1970;117(538):287–292. PubMed CrossRef
- Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? a meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61(7):631–637. PubMed CrossRef
- Kim JM, Stewart R, Kim SW, et al. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br J Psychiatry. 2008;192(4):268–274. PubMed CrossRef
- Dimopoulos N, Piperi C, Salonicioti A, et al. Correlation of folate, vitamin B12 and homocysteine plasma levels with depression in an elderly Greek population. Clin Biochem. 2007;40(9-10):604–608. PubMed CrossRef
- Sachdev PS, Parslow RA, Lux O, et al. Relationship of homocysteine, folic acid and vitamin B12 with depression in a middle-aged community sample. Psychol Med. 2005;35(4):529–538. PubMed CrossRef
- Bottiglieri T, Reynolds EH, Laundy M. Folate in CSF and age. J Neurol Neurosurg Psychiatry. 2000;69(4):562. PubMed CrossRef
- Petridou ET, Kousoulis AA, Michelakos T, et al. Folate and B12 serum levels in association with depression in the aged: a systematic review and meta-analysis. Aging Ment Health. 2016;20(9):965–973. PubMed CrossRef
- Bottiglieri T, Hyland K, Laundy M, et al. Folate deficiency, biopterin and monoamine metabolism in depression. Psychol Med. 1992;22(4):871–876. PubMed CrossRef
- Placidi GP, Oquendo MA, Malone KM, et al. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry. 2001;50(10):783–791. PubMed CrossRef
- Goodwin FK, Post RM. 5-hydroxytryptamine and depression: a model for the interaction of normal variance with pathology. Br J Clin Pharmacol. 1983;15(suppl 3):393S–405S. PubMed CrossRef
Kaufman S. Some metabolic relationships between biopterin and folate: implications for the “methyl trap hypothesis”. Neurochem Res. 1991;16(9):1031–1036. PubMed CrossRef
Taylor MJ, Carney S, Geddes J, et al. Folate for depressive disorders. Cochrane Databases Syst Rev. 2003;2003(2):CD003390. PubMed CrossRef
Wesson VA, Levitt AJ, Joffe RT. Change in folate status with antidepressant treatment. Psychiatry Res. 1994;53(3):313–322. PubMed CrossRef
- Alpert M, Silva RR, Pouget ER. Prediction of treatment response in geriatric depression from baseline folate level: interaction with an SSRI or a tricyclic antidepressant. J Clin Psychopharmacol. 2003;23(3):309–313. PubMed CrossRef
- Coppen A, Chaudhry S, Swade C. Folic acid enhances lithium prophylaxis. J Affect Disord. 1986;10(1):9–13. PubMed CrossRef
- Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000;60(2):121–130. PubMed CrossRef
- Food and Drug Administration. Authors food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Final Rule. 21 CFR Parts 136, 137, and 139. Fed Regist. 1996;61:8781–8789.
- Guaraldi GP, Fava M, Mazzi F, et al. An open trial of methyltetrahydrofolate in elderly depressed patients. Ann Clin Psychiatry. 1993;5(2):101–105. PubMed CrossRef
- Di Palma C, Urani R, Argicola R, et al. Is methylfolate effective in relieving major depression in chronic alcoholics? a hypothesis of treatment. Curr Ther Res. 1994;55(5):559–568. CrossRef
- Ginsberg LD, Oubre AY, Daoud YA. L-methylfolate Plus SSRI or SNRI from treatment initiation compared to SSRI or SNRI monotherapy in a major depressive episode. Innov Clin Neurosci. 2011;8(1):19–28. PubMed
- Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267–1274. PubMed CrossRef
- Shelton RC, Sloan Manning J, Barrentine LW, et al. Assessing effects of L-methyfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):PCC.13m01520. PubMed CrossRef
- Passeri M, Cucinotta D, Abate G, et al. Oral 5′-methyltetrahydrofolic acid in senile organic mental disorders with depression: results of a double-blind multicenter study. Aging (Milano). 1993;5(1):63–71. PubMed CrossRef
- Godfrey PS, Toone BK, Carney MW, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392–395. PubMed CrossRef
- Alpert JE, Mischoulon D, Rubenstein GE, et al. Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry. 2002;14(1):33–38. PubMed CrossRef
- Heslin KC, Elixhauser A, Steiner CA. Hospitalizations involving mental and substance use disorders among adults, 2012: Statistical Brief #191. In: Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Research and Quality; June 2015.
- Bell IR, Edman JS, Marby DW, et al. Vitamin B12 and folate status in acute geropsychiatric inpatients: affective and cognitive characteristics of a vitamin nondeficient population. Biol Psychiatry. 1990;27(2):125–137. PubMed CrossRef
- Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983-1983;17(1):37–49. PubMed CrossRef
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top