Prim Care Companion CNS Disord 2021;23(6):20l02873
To cite: Naguy A. Levomilnacipran for negative symptom domain schizophrenia. Prim Care Companion CNS Disord. 2021;23(6):20l02873.
To share: https://doi.org/10.4088/PCC.20l02873
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Child/Adolescent Psychiatry, Al-Manara CAP Centre, Kuwait Centre for Mental Health, Shuwaikh, Kuwait
*Corresponding author: Ahmed Naguy, MBBch, MSc, Department of Child/Adolescent Psychiatry, Al-Manara CAP Centre, Kuwait Centre for Mental Health, Jamal Abdul-Nassir St, Shuwaikh, Kuwait ([email protected]).
Although the positive symptom domain comprises the most vivid and conspicuous symptoms of schizophrenia and is the chief cause of referral for treatment, the negative symptom domain is pervasive but sometimes invisible and especially difficult to treat. Here, the case of a patient with chronic schizophrenia is reported wherein add-on levomilnacipran brought about striking improvement in the negative symptom domain.
Case Report
Mr A was a 24-year-old Kuwaiti man diagnosed with schizophrenia disorder at age 19 years who was currently maintained on paliperidone palmitate 234 mg intramuscular monthly and procyclidine 15 mg/d. He dropped out of college after he was first hospitalized for his illness. He had a heavy genetic load. He was homebound and lived with his octogenarian father on social allowance by Public Authority of the Disabled. As per his chart, he had erratic attendance for outpatient follow-ups and was no longer engaging in rehabilitative services. He was recently assessed at his father’s request to possibly shift him to paliperidone 3 times/month instead for more convenience. Also, his father was concerned that Mr A had recently experienced diminished appetite and terminal insomnia. On mental status examination, he had marginal grooming and self-hygiene and was unkempt and disheveled with a musty odor. He was skinny, was detached with bland facial expression, and was slavering at times. He had an attitude d’impénétrabilité (or lack of rapport) with a flat affect. He was taciturn with laconic hesitant speech and sterile thought content. He self-rated his mood as 3/10, but for no good reason, for the past couple of weeks, with shallow affect and occasional ululations. He denied ideations of deliberate self-harm or unusual perceptual experiences. He scored 23 on the 4-item Negative Symptom Assessment (NSA-4).1 Also, he scored 1 recalled word + abnormal Clock Drawing Test on the Mini-Cog.2 The urinary toxicology screen was negative. His latest laboratory workup was within normal limits apart from asymptomatic hyperprolactinemia (700 U) and borderline high low-density lipoprotein cholesterol at 160 mg/dL. Adjuvant off-label use of levomilnacipran extended release was proposed, was commenced at 20 mg/d, and was uptitrated to 60 mg/d over 4 days. Three weeks later, marked improvement in negative and cognitive symptom domains was noticed and confirmed on the NSA-4 and Mini-Cog (down to 10 and 3 recalled words + abnormal Clock Drawing Test, respectively). This improvement was well-sustained at 16 weeks, at the time of writing this report, with great tolerability. No drug-drug interactions were experienced.
Discussion
Levomilnacipran, an l-enantiomer of milnacipran, is a serotonin-norepinephrine reuptake inhibitor antidepressant with an S:N ratio of 1:2. It is a substrate for cytochrome P450 3A4. It has a therapeutic potential for Alzheimer’s disease due to BACE-1 (beta-site amyloid precursor protein cleaving enzyme-1) inhibitory activity. Elimination half-life is 12 hours, allowing for once/day dosing. It comes in extended-release formulation. The dose range is 40–120 mg/d.3
It seems that boosting noradrenergic (NE) drive by levomilnacipran accounts for its procognitive effects. Subsequent disinhibition of dopaminergic projections to the dorsolateral prefrontal cortex corrects the dopamine hypofrontality underlying the negative symptoms.4 Moreover, decreased NE in patients with chronic schizophrenia is well-documented in the literature, and psychotropics acting primarily to increase NE (eg, milnacipran, mirtazapine, duloxetine, atomoxetine) were reported to mitigate negative symptoms,5–8 although evidence supporting use of antidepressants for the primary negative symptom domain in schizophrenia is generally lacking.9
Recognition of the negative symptom domain in schizophrenia dates to the earliest descriptions by Kraepelin and Bleuler10 and includes the popular “As”: Affective flattening, Alogia, Avolia, Abulia, Anhedonia, Asociality, and Attentional impairment.
A distinction has been made between primary (enduring or deficit state) and secondary (phasic) negative symptoms. These phasic negative symptoms might be secondary to positive symptoms, extrapyramidal side effects related to conventional antipsychotic treatment, depression, substance use, catatonia, neurologic insult, or institutionalization and social disadavantage.11 Negative symptoms are notably prominent in deficit state and so-called simple schizophrenia.
Negative symptoms are commonly assessed using instruments like the Scale for the Assessment of Negative Symptoms (SANS),12 which covers most of the negative domain. It should be noted, however, that items like attentional impairment, inappropriate affect, and poverty of content of speech covered in the SANS are currently envisioned as a disorganized symptom domain rather than a bona fide negative symptom domain. The NSA-4 is a simple tool that rapidly and effectively assesses the negative domain.
There seems to be a relationship that exists between negative and cognitive symptom domains in schizophrenia, and both substantially impact functional recovery. However, there is no clear link between negative symptoms and specific cognitive deficits, suggesting they might represent semiautonomous disease processes.13
The genuine negative symptom domain appears to segregate into 2 underlying factors: diminished expression (comprising affective flattening and poverty of speech) and amotivation (comprising avolia and anhedonia/asociality). Hedonic deficits in schizophrenia have been characterized by anticipatory anhedonia (with intact consummatory counterpart).14 Review of the literature suggests that the negative symptom domain, especially the motivational deficits, is critically related to functional outcome in schizophrenia, while also mediating the impact of cognitive deficits on functional recovery.15,16
Negative symptom characteristics of schizophrenia have been identified in at-risk mental state and prodroma populations. Less severe symptoms have been shown in schizoaffective disorder. There is also some limited evidence of intact hedonic capacity and concurrent motivational deficits.
Negative symptoms have been linked to hypofrontality and mapped to prefrontal cortical and ventral striatal areas.17 The negative symptom domain is generally believed to represent the phenotypic expression of an underlying hypodopaminergic cortical state.
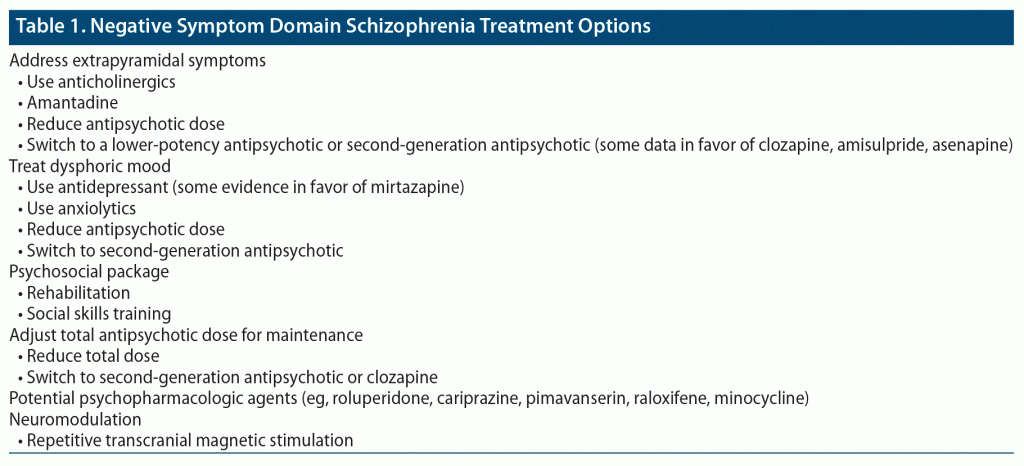
Prominent negative symptoms affect approximately 40% of patients with schizophrenia. Primary negative symptoms have been described in relation to the positive symptom domain as relatively synchronized (simultaneously progressive) versus relatively desynchronized (independently progressive) trajectories in the long run.18 Some psychopharmacologic tips to mitigate the negative symptom domain19 are provided in Table 1.
This case, to the best of my knowledge, is one of the earliest to report on levomilnacipran efficacy for the negative symptom domain in schizophrenia. Large well-conducted studies are needed to replicate this finding.
Published online: December 9, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Consent was received from the patient to publish this case report, and information has been de-identified to protect anonymity.
References (19)

- Alphs L, Morlock R, Coon C, et al. The 4-item Negative Symptom Assessment (NSA-4) instrument: a simple tool for evaluating negative symptoms in schizophrenia following brief training. Psychiatry (Edgmont). 2010;7(7):26–32. PubMed
- Borson S, Scanlan JM, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451–1454. PubMed CrossRef
- Bruno A, Morabito P, Spina E, et al. The role of levomilnacipran in the management of major depressive disorder: a comprehensive review. Curr Neuropharmacol. 2016;14(2):191–199. PubMed CrossRef
- Sabe M, Kirschner M, Kaiser S. Prodopaminergic drugs for treating the negative symptoms of schizophrenia: systematic review and meta-analysis of randomized controlled trials. J Clin Psychopharmacol. 2019;39(6):658–664. PubMed CrossRef
- Nakanishi S, Kunugi H, Takahashi T. Efficacy of milnacipran for depressive symptoms in schizophrenia spectrum disorders. Psychiatry Clin Neurosci. 2004;58(2):226–227. PubMed CrossRef
- Moodliar S, Naguy A, Elsori DH. Add-on mirtazapine to clozapine-responsive early-onset schizophrenia. Psychiatry Res. 2020;284:112701. PubMed CrossRef
- Pridmore S, Naguy A, Moodliar-Rensburg S, et al. Advantageous add-on duloxetine to aripiprazole-responsive early-onset schizophrenia. J Clin Psychopharmacol. 2021;41(1):91–92. PubMed
- Naguy A, Alamiri B, Khraibut B. Weight loss during atomoxetine add-on to clozapine-responsive schizophrenia. Am J Ther. 2018;25(6):e774–e775. PubMed CrossRef
- Hinkelmann K, Yassouridis A, Kellner M, et al. No effects of antidepressants on negative symptoms in schizophrenia. J Clin Psychopharmacol. 2013;33(5):686–690. PubMed CrossRef
- Hoenig J. The concept of schizophrenia. Kraepelin-Bleuler-Schneider. Br J Psychiatry. 1983;142(6):547–556. PubMed CrossRef
- Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–534. PubMed CrossRef
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry suppl. 1989;155(S7):49–58. PubMed CrossRef
- Bagney A, Dompablo M, Santabárbara J, et al. Are negative symptoms really related to cognition in schizophrenia? Psychiatry Res. 2015;230(2):377–382. PubMed CrossRef
- Naguy A, Alwetayan S, AlKhadhari S. Anhedonia as a transdiagnostic construct. Asian J Psychiatr. 2020;48:101604. PubMed CrossRef
- Montemagni C, Castagna F, Crivelli B, et al. Relative contributions of negative symptoms, insight, and coping strategies to quality of life in stable schizophrenia. Psychiatry Res. 2014;220(1–2):102–111. PubMed CrossRef
- Naguy A, Al-Rabaie A. Suicidality and survivability in schizophrenia. J Nerv Ment Dis. 2017;205(7):585. PubMed CrossRef
- Martino DJ, Bucay D, Butman JT, et al. Neuropsychological frontal impairments and negative symptoms in schizophrenia. Psychiatry Res. 2007;152(2-3):121–128. PubMed CrossRef
- Austin SF, Mors O, Budtz-Jørgensen E, et al. Long-term trajectories of positive and negative symptoms in first-episode psychosis: a 10-year follow-up study in the OPUS cohort. Schizophr Res. 2015;168(1–2):84–91. PubMed CrossRef
- Buchanan RW, Panagides J, Zhao J, et al. Asenapine versus olanzapine in people with persistent negative symptoms of schizophrenia. J Clin Psychopharmacol. 2012;32(1):36–45. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top