ABSTRACT
Objective: People with bipolar disorder (BD) often have difficulty with medication adherence. This pilot trial combined a behavioral customized adherence enhancement (CAE) approach with long-acting injectable (LAI) antipsychotic medication and assessed effects on adherence, BD symptoms, and functional status.
Methods: This 6-month prospective, uncontrolled trial of the intervention (CAE with LAI) in 30 poorly adherent individuals with BD assessed adherence using the Tablets Routine Questionnaire (TRQ) and symptoms using the Brief Psychiatric Rating Scale (BPRS), Young Mania Rating Scale (YMRS), Hamilton Depression Rating Scale (HDRS), and Clinical Global Impressions (CGI). Functioning was assessed via the Social and Occupational Functioning Assessment Scale (SOFAS) and Global Assessment of Functioning (GAF). Assessments were conducted at screening, baseline, week 12, and week 24 (6 months). The LAI was aripiprazole once monthly. The study was conducted between April 2018 and May 2020.
Results: The mean age of the sample was 49.5 years (SD = 9.3), and 56.7% were Black. Nine individuals (30%) terminated the study prematurely, 1 due to side effects (tremor). The mean LAI dose was 314.3 mg (SD = 96.4). The proportion of missed medications in the past week (mean TRQ) from screen to 24 weeks significantly improved from 50.1% (SD 24.8) to 16.9% (SD = 27.0) (P < .001), and past month TRQ improved from 40.6% (SD = 23.8) to 19.2% (SD = 24.0) (a trend for significance, P = .0599). TRQ change from baseline to 24 weeks was not significant. There were significant improvements on the BPRS (P < .001), MADRS (P = .01), YMRS (P < .001), CGI (P < .001), SOFAS (P < .001), and GAF (P < .001).
Conclusion: A personalized intervention to address adherence barriers combined with LAI can improve recovery outcomes in high-risk individuals with BD.
Trial Registration: ClinicalTrials.gov Identifier: NCT03408873
Prim Care Companion CNS Disord 2021;23(5):20m02888
To cite: Sajatovic M, Levin JB, Ramirez LF, et al. Long-acting injectable antipsychotic medication plus customized adherence enhancement in poor adherence patients with bipolar disorder. Prim Care Companion CNS Disord. 2021;23(5):20m02888.
To share: https://doi.org/10.4088/PCC.20m02888
© Copyright 2021 Physicians Postgraduate Press, Inc.
aNeurological and Behavioral Outcomes Center, Case Western Reserve University School of Medicine and University Hospitals Cleveland Medical Center, Cleveland, Ohio
bDepartment of Psychiatry, Case Western Reserve University School of Medicine and University Hospitals Cleveland Medical Center, Cleveland, Ohio
cDepartment of Population and Quantitative Health Sciences, Case Western Reserve University School of Medicine, University Hospitals Cleveland Medical Center, Cleveland, Ohio
*Corresponding author: Martha Sajatovic, MD, Department of Psychiatry, W.O. Walker Bldg, 7th Floor, 10524 Euclid Ave, Cleveland, OH 44106 ([email protected]).
Poor medication adherence in bipolar disorder (BD) is associated with relapse and poor outcomes.1,2 A growing body of literature focuses on clinical approaches to address poor adherence.3 Behavioral programs that target patient-specific adherence barriers may lead to substantial outcome gains.4–8
Another way to optimize treatment adherence is by using medication delivery approaches that maximize ease of use.9,10 The long-acting injectable (LAI) antipsychotic medications aripiprazole LAI and risperidone LAI are currently US Food and Drug Administration (FDA) approved for the treatment of BD and may represent an opportunity to address adherence barriers such as forgetting to take daily oral tablets. However, in spite of the adherence advantages for LAIs, simply switching individuals to an LAI may not be enough to sustain long-term behavioral change. Additionally, many people with BD are on multiple psychotropic medications,11 some of which are not available as LAIs.
Adherence is a multicomponent process that involves having knowledge of what is needed to manage a chronic health condition, organizational resources for self-management, ability to communicate with health care professionals effectively, and understanding the impact of substance use on adherence. Problems with any of these components can impede adherence.12 An intervention that combines LAI with a personalized barrier-focused behavioral approach can improve adherence, symptoms, and functioning in people with primary psychotic disorders.6,13 This pilot trial of a brief behavioral approach called customized adherence enhancement (CAE) combined with LAI (CAE-L) assessed effects on adherence, BD symptoms, and functional status in a high-risk sample of patients with BD. We expected that CAE-L would be well tolerated and lead to clinical improvement.
METHODS
Overview
This 6-month, prospective, single-arm intervention trial tested the effects of CAE-L in 30 poorly adherent patients with type 1 or type 2 BD (ClinicalTrials.gov Identifier: NCT03408873). The study was conducted between April 2018 and May 2020. The monthly LAI was cross-tapered and substituted for oral antipsychotics in individuals on an oral antipsychotic or added to other currently prescribed BD medication treatments for individuals not on oral antipsychotics. CAE was delivered by a trained social worker following a detailed curriculum and delivered in the same clinical visit as the LAI. Outcomes were assessed at screening, baseline, week 12, and week 24 (6 months). Primary outcomes were change in adherence as identified by the Tablets Routine Questionnaire (TRQ)14,15 and LAI injection frequency at week 24. Secondary outcomes included BD symptoms, functional status, and adherence attitudes. LAI tolerability was assessed via self-reported side effects and standardized scales. Exploratory assessments included health resource use, adherence barriers, substance use, and neurocognition. Primary and most secondary outcome measures were assessed at screening, baseline, week 12, and week 24. Side effects were assessed at each clinical visit.
Sample
Participants were adults aged ≥ 18 years with type 1 or type 2 BD confirmed with the Mini-International Neuropsychiatric Interview (MINI).16 Individuals were recruited from an academic medical center and via outreach to community settings including community mental health clinics (CMHCs). Enrolled participants had self-reported adherence problems as identified by the TRQ (≥ 20% missed BD medications in past week or past month), a screening Brief Psychiatric Rating Scale (BPRS)17 score ≥ 36, and willingness to take LAI and were in treatment at a CMHC or other clinical setting. Individuals already on an LAI, those with known intolerance or resistance to aripiprazole, prior or current treatment with clozapine, unstable medical conditions, or physical dependence on substances were excluded. The CAE intervention was available only in English, and questionnaires required the ability to read English. All participants provided written informed consent, and the study was approved by the local institutional review board.
LAI Antipsychotics
Oral aripiprazole is effective in the treatment of patients with BD as an acute antimanic agent and for maintenance treatment.18 A monthly intramuscular (IM) depot formulation has been demonstrated to reduce BD relapse.19,20 The FDA has approved aripiprazole once monthly (Abilify Maintena), the LAI used in this study, as maintenance monotherapy for the treatment of type 1 BD. Abilify Maintena is not approved for treatment of BD depression. Medication dosing for this study followed package insert recommendations (www.otsuka-us.com). Individuals who were on an oral antipsychotic drug at baseline were cross-tapered such that the oral drug was titrated down and discontinued as appropriate once the LAI was started. If the individual was not on an antipsychotic drug at baseline, the LAI was added to the existing regimen. For patients who had never received aripiprazole, there was a brief oral tolerance testing of up to 14 days. Individuals who tolerated oral tolerance testing then received 400 mg of LAI administered IM (a lower dosage was used if the research psychiatrist had concerns regarding tolerance or reduced drug metabolism), and oral aripiprazole was continued for an additional 14 days and then stopped. Injections were given monthly for 6 months, with LAI dosing adjustments at the discretion of the research psychiatrist.
Concomitant Medications
As multidrug treatment is common in BD, patients continued other psychotropic maintenance treatments defined as traditional mood-stabilizing drugs (lithium, valproate, or lamotrigine) or antidepressants prescribed for at least 1 month at a stable dosage. Hypnotic drugs for sleep prescribed for at least 1 month prior to enrollment were also continued.
CAE Intervention
The CAE program is a brief, practical intervention consisting of a series of up to 4 psychosocial treatment modules based on the individual’s unique adherence barriers: (1) psychoeducation on BD medications, (2) communication with providers, (3) strategies to enhance medication routines, and (4) targeting substance use problems with modified motivational enhancement therapy. These CAE modules are derived from existing evidence-based approaches in BD.21–26 Each module can be combined with other modules as determined by a screening adherence barrier assessment. For this study, CAE was delivered in 7 sessions (baseline and at each monthly visit thereafter) lasting approximately 30–60 minutes.
Study Hypotheses and Intervention Target Engagement Conceptual Model
We hypothesized that at 24-week follow-up, patients on CAE-L would have significant improvement in adherence as measured by the TRQ and that LAI injection frequency would be ≥ 80%, a common benchmark for “acceptable” adherence.27 As secondary hypotheses, we expected reduction in BD symptoms, improved functioning, and better treatment attitudes. We also explored whether adherence barriers would be associated with adherence change.
Measures
Adherence behaviors, attitudes, and barriers. Adherence behavior (medication taking) was assessed for each BD maintenance medication using the TRQ, which derives a proportion (%) of days with any missed medication doses in the last week and last month. TRQ scores range from perfect adherence (0% missed) to missing all medication (100% missed). An average TRQ was calculated for individuals on > 1 BD medication.14,15,28 LAI injection frequency was calculated as the proportion of actual injections to those the individual would have received with perfect attendance (counted as “adherent” if administered within 7 days of scheduled administration). The 10-item Drug Attitude Inventory (DAI)29 and the Attitude Toward Medication Questionnaire (AMSQ)14,30 assessed attitudes toward medication. The DAI and AMSQ were assessed at study screen and at week 12 and week 24.
The adherence barriers of inadequate BD knowledge, unstable lifestyle routines, suboptimal communication with providers, and substance use were assessed with the Oxford Bipolar Knowledge Questionnaire (OBQ),31 the Self-Report Habit Index (SRHI),32 the Physician Communication Style (PCS),33 and the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES 8A),34 respectively.
Safety evaluations. Safety evaluations at baseline and week 24 included a comprehensive metabolic panel, lipids, complete blood count (CBC) with differential, thyroid function, and pregnancy testing for women. Electrocardiogram (EKG) was conducted at baseline, week 4, and week 24. Vital signs, weight, and reported side effects were collected at each study visit as were standardized measures of extrapyramidal symptoms including the Simpson Angus Scale (SAS),35 the Barnes Akathisia Scale (BARS),36 the Abnormal Involuntary Movement Scale (AIMS),37 and the Extrapyramidal Symptoms Rating Scale-Abbreviated version (ESRS-A).38
BD symptoms, comorbidity. Symptoms were assessed with the BPRS, Young Mania Rating Scale (YMRS),39 Montgomery-Asberg Depression Rating Scale (MADRS),40 and Clinical Global Impressions (CGI).36 Substance use was evaluated with the Alcohol Use Disorders Identification Test-Self-Report Version (AUDIT)41 and Drug Abuse Screening Test (DAST-10).42 We categorized an individual as having substance use problems if they met diagnostic criteria for substance use disorder comorbidity on the MINI or had a score > 8 on the AUDIT or a score > 6 on the DAST-10.
Functioning and neurocognition. Functional status was evaluated using the Social and Occupational Functioning Assessment Scale (SOFAS)43 and the Global Assessment of Functioning (GAF).44 Neurocognitive assessments were the Trail Making Test Parts A and B45 and Animal Fluency Test46 to assess for executive functioning and semantic verbal fluency, respectively.
Additional exploratory assessments. We assessed health resource use in the 6-month study period and in the 6-month period prior to study enrollment. We assessed CAE treatment acceptability and satisfaction using a Likert scale.
Data Analysis
Statistical analysis was conducted with the Statistical Analysis System (SAS Institute, Cary, North Carolina). Descriptive statistics (means and standard deviations or counts and percentages) were generated for all outcome measures and sociodemographic attributes using complete cases. We compared for the primary and secondary outcomes across 2 sets of time points: (1) difference between screen and week 24 and (2) difference between baseline and week 24. A nonparametric Wilcoxon signed rank test compared these repeated measurements (matched on participant) between these time points to determine whether the population medians differed. Given the modest sample size and that missing observations for a specific outcome measure were not imputed, the Ns for each comparison are reported. Mean LAI injection frequency and 95% confidence intervals were also estimated. A 2-sided α of .05 was considered statistically significant.
RESULTS
Study Flow and Sample
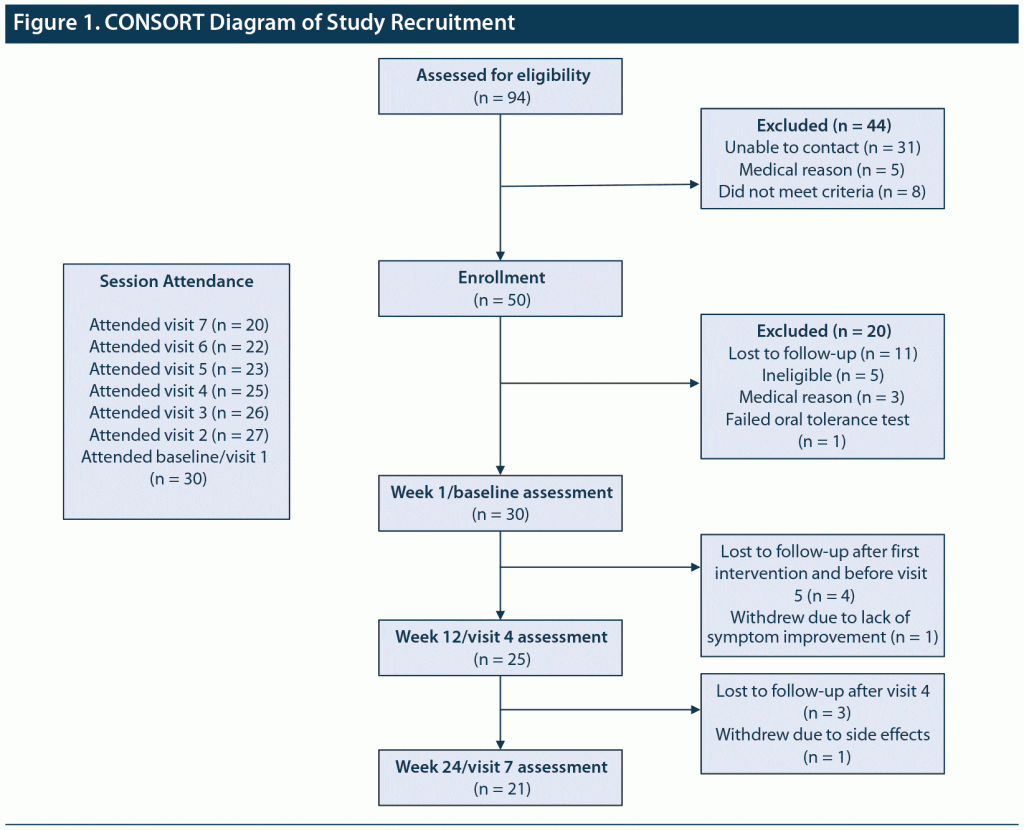
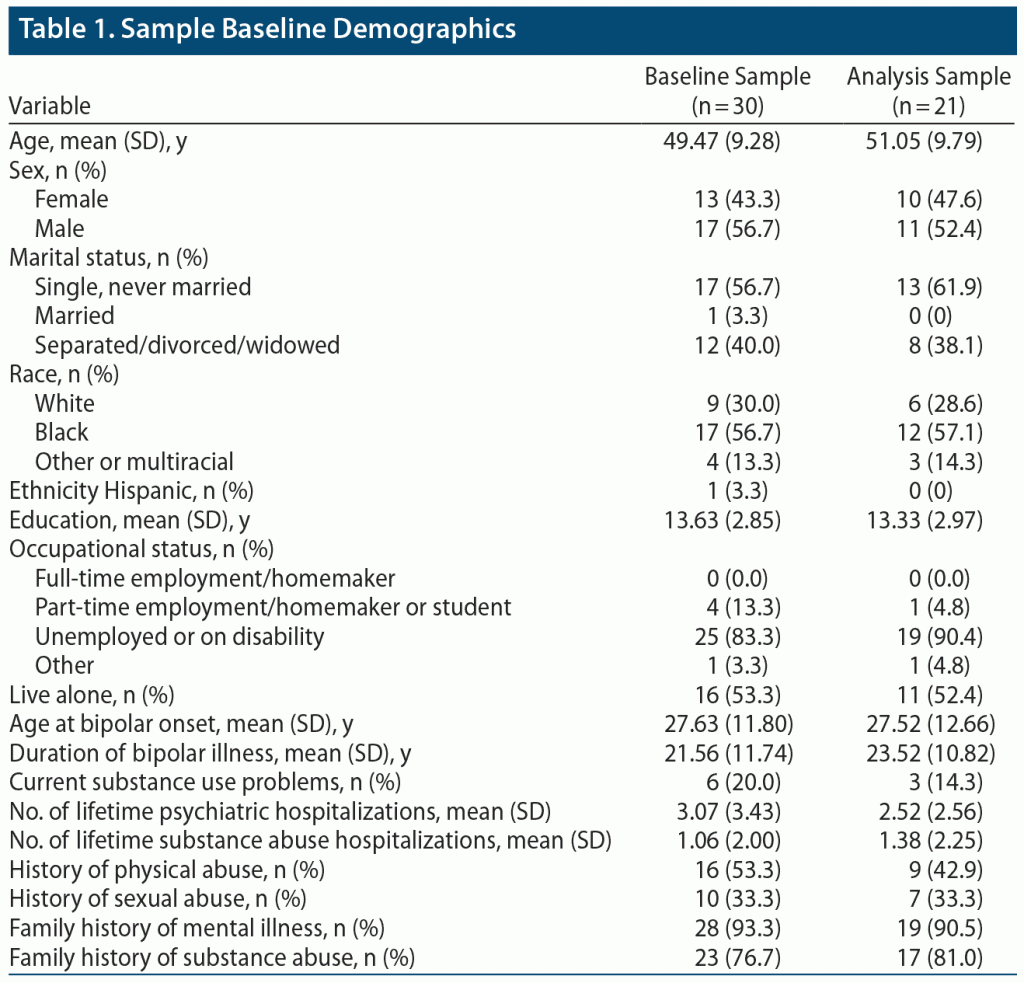
Figure 1 is a CONSORT diagram showing study enrollment and retention. Table 1 illustrates baseline clinical variables. The sample mean age was 49.5 years (SD = 9.3), with a majority Black (56.7%), unemployed (83.3%), and living alone (53.3%). Patients had a mean of 3 past psychiatric hospitalizations for BD. Rates of past physical and sexual abuse were high, and nearly all individuals had a family history of mental illness or substance abuse. There were 6 individuals (20%) with current substance use problems, with the main substances used (alone or in combination) being alcohol (n = 6), marijuana (n = 3), and cocaine (n = 1).
LAI
The mean endpoint dose of aripiprazole LAI was 314.29 mg (SD = 96.36, range, 100–400 mg).
Concomitant Medication
Individuals were prescribed a baseline average of 1.48 (SD = 0.63) BD medications. This included antipsychotics (n = 22, 73.3%), lithium (n = 3, 10.0%), anticonvulsant mood stabilizers (n = 13, 43.3%), and other regularly scheduled psychotropic medications (n = 3, 10.0%). As-needed medications were not included in the count of concomitant medications. There were 14 individuals, 67% of the total sample of 21 who completed the trial, who were on LAI monotherapy at study endpoint.
CAE Components
Based on the screening barriers assessment, 23/30 (76.7%) individuals were assigned to receive the CAE module that addressed inadequate understanding of BD (psychoeducation), 28/30 (93.3%) the module that addressed lack of medication-taking routines (medication routines), 24/30 (80%) the module that addressed poor communication with care providers (communication), and 26/30 (86.7%) the module that addressed substance use (modified motivational interviewing). The substance use module was assigned if individuals had current substance use problems or if they felt it would be helpful for them to receive this module based on substance problems in the past. Just over half (n = 17, 56.7%) were assigned to all 4 CAE modules, 8 (26.7%) to 3 modules, 4 (13.3%) to 2 modules, and 1 (3.3%) to a single module. For individuals that attended at least 1 CAE session, the mean session attendance (total maximum of 7 visits) was 6.8 (SD = 0.67).
Dropouts and Safety
There were 9 individuals (30.0%) who terminated the study prematurely. Reasons for dropout included 7 (23.3%) lost to follow-up, 1 (3.3%) due to lack of BD symptom improvement, and 1 (3.3%) due to adverse effects (tremor). There were no serious adverse events.
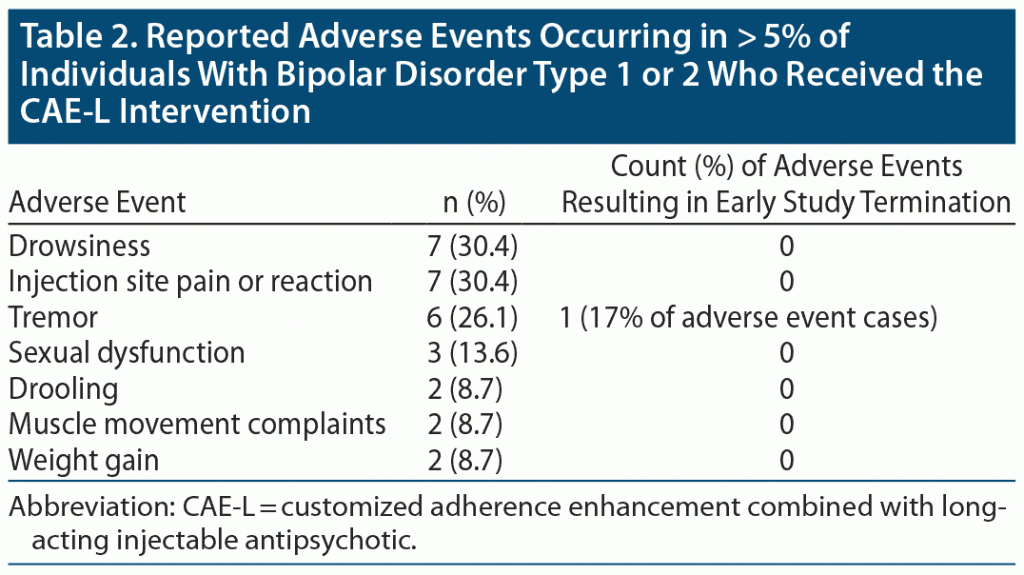
Table 2 illustrates side effects experienced by > 5% (n ≥ 2) of study participants. The most common side effects were drowsiness (30.4%) and injection site pain or reaction (30.4%). There were no serious or sustained injection site reactions. There were no significant changes on the AIMS, SAS, BARS, or ESRS-A at week 24. Mean weight gain was 2.05 kg (4.51 lb) (SD = 6.71 kg [14.79 lb]), with 5 (22.7%) individuals gaining > 7% of their body weight. There was no significant change in serum total cholesterol, low-density lipoprotein, or triglyceride levels between baseline and week 24. High-density lipoprotein levels decreased by a mean of 4.87 (SD = 7.19).
Efficacy, Intervention Target Engagement
Table 3 illustrates change in primary and secondary outcomes. CAE-L was associated with excellent adherence to LAI (100% of individuals received injection within 1 week of the scheduled time). At screen, individuals missed a mean of 50.1% (SD = 24.8%) of prescribed oral medication in the past week and 40.6% (SD = 23.8%) of medication in the past month. At baseline, individuals were missing 37.5% (SD = 30.0) and 29.4% (SD = 21.6%) of prescribed oral medication in the past week and past month, respectively. The proportion of missed medications in the past week (mean TRQ) from screen to 24 weeks significantly improved from 50.1% (SD = 24.8) to 16.9% (SD = 27.0) (P < .001), and past month TRQ improved from 40.6% (SD = 23.8) to 19.2% (SD = 24.0) (a trend for significance, P = .0599). TRQ change from baseline to 24 weeks was not significant (past week: P = .12, past month: P = .63). We note that only 11 participants were receiving oral maintenance medication for BD in addition to LAI at endpoint, and TRQ data were based on this subset.
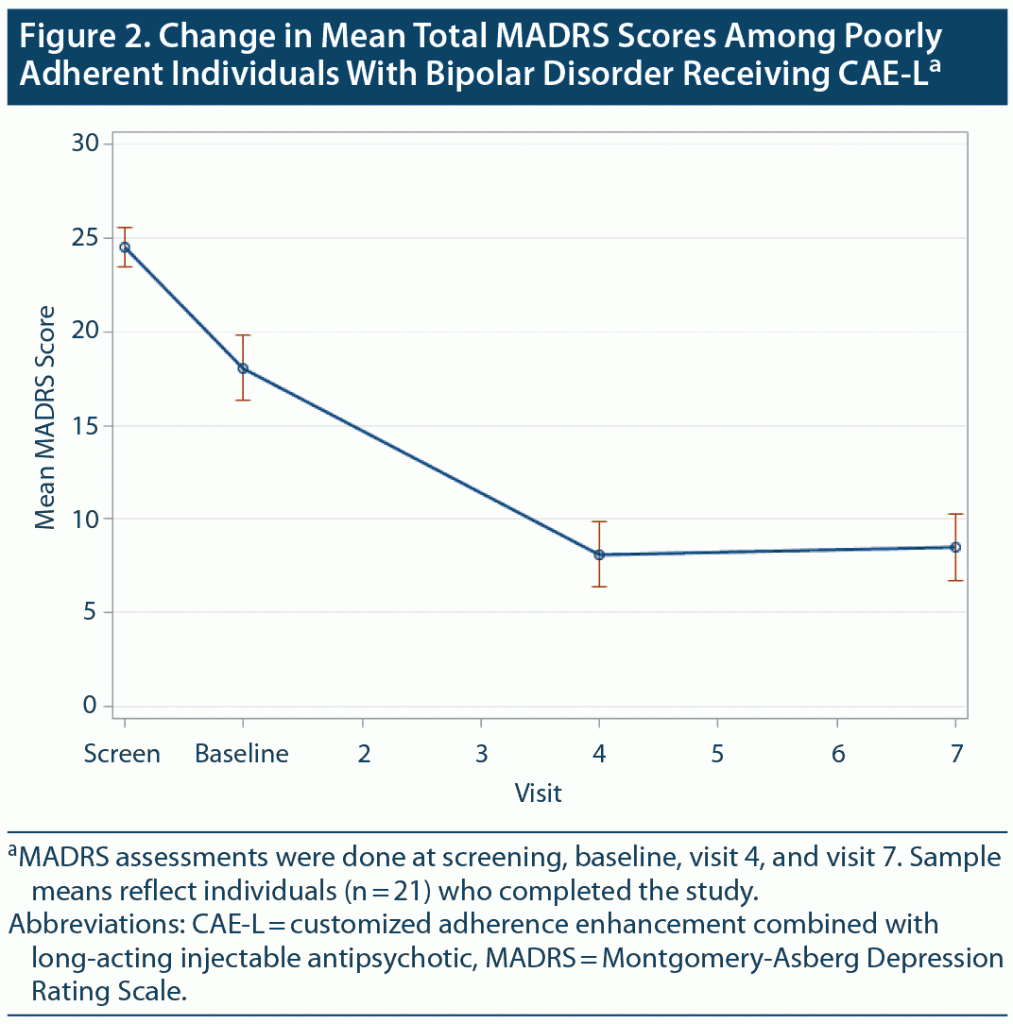
At baseline, individuals were relatively symptomatic, mostly with BD depressive symptoms. As noted in Table 3, baseline MADRS total was 18.1 (SD = 9.4), while baseline YMRS was 10.4 (SD = 5.6). From baseline to week 24, there were significant decreases in BD symptoms and global psychopathology as assessed by total scores on the BPRS (P < .001), MADRS (P = .01), YMRS (P < .001), and CGI (P < .001). Figure 2 shows change in mean MADRS scores. Functioning measured by the SOFAS and GAF was significantly improved from baseline to week 24 (P < .001).
As shown in Table 3, there was a significant improvement in BD knowledge measured by the OBQ and in medication routines management measured by the SRHI (P < .001 for both) and the SOCRATES 8A (P = .02). With respect to the relationship between the intervention target engagement, we did not find that change (reduction) in barriers (OBQ, SRHI, PCS, and SOCRATES 8A) was correlated with change in TRQ (OBQ: P = .68, SRHI: P = .21, PCS: P = .47, SOCRATES 8A: P = .99). We also explored the association between TRQ and BD symptoms (BPRS, MADRS, YMRS, CGI) and found that BPRS and MADRS scores were significantly and positively associated with TRQ scores (more missed drug was associated with higher levels of symptom severity) (BPRS: P = .006, MADRS: P = .0446).
With respect to health resource use, there was no significant reduction in hospitalizations. It must be noted that hospitalizations were low to begin with, and 73.9% of individuals had no hospitalizations in the 6 months prior to study entry. There were also no significant changes in any of the measures of neurocognition (data not shown).
Finally, 23 individuals provided input on CAE treatment acceptability. Most (20/21, 95.6%) individuals agreed or strongly agreed that CAE covers all or most of the important issues, while the number of sessions were perceived as just about right in 17/23 (73.9%), too long in 2/23 (8.7%), and too short in 4/23 (17.4%). Most (21/23, 91.3%) individuals strongly agreed or agreed that the benefit of attending CAE exceeded the burden. All individuals who answered the LAI acceptability survey (n = 21) strongly agreed or agreed that LAI was helpful, and 14/22 (63%) stated they planned to continue on LAI post study.
DISCUSSION
In this pilot trial evaluating combining aripiprazole LAI with a psychosocial intervention to enhance treatment adherence, self-reported adherence behaviors as measured by the TRQ improved from study screen to week 24. Adherence change from baseline to week 24 was not significant. BD symptoms, including BPRS, MADRS, YMRS, and CGI, significantly improved over 24 weeks. Functional measures on the GAF and SOFAS also improved. Findings from this prospective uncontrolled trial generally align with findings from a previous application of CAE-L in poorly adherent patients with schizophrenia or schizoaffective disorder.6,13
There are a few points that highlight the clinical relevance of this pilot study in spite of methodological limitations. First, the study specifically targeted and enrolled individuals with known poor adherence. This is a subgroup of individuals not typically enrolled in treatment studies, despite that they are representative of many individuals in real-world treatment settings.2,47,48 Additionally, the sample was quite diverse, with approximately 70% of individuals self-identifying as Black or multiracial. Our study findings are consistent with reports noting that minorities with BD may have challenges with medication adherence.49 The sample enrolled in this study also had significant functional impairment, with most (83%) on occupational disability or unemployed. Over half lived alone. A systematic review of antipsychotic medication adherence in 38 studies, conducted in a total of 51,796 patients and including individuals with BD, found that younger age, substance abuse, poor insight, cognitive impairment, low level of education, minority ethnicity, poor therapeutic alliance, experience of barriers to care, high intensity of delusional symptoms and suspiciousness, and low socioeconomic status are the main risk factors for nonadherence.47 In general, the sociodemographic and clinical characteristics of our study sample align with features known to be present among poorly adherent BD patients.48,49
In spite of substantial disadvantages with respect to clinical factors and limited social supports, patients receiving CAE-L had symptomatic and functional improvement over 6 months. The CAE intervention, delivered by a social worker during the same clinical visit that LAI was administered, was highly acceptable to patients. Delivery by social workers (as opposed to more specialized psychiatric staff) may optimize the potential for future broader scale-up in clinical settings. Additionally, CAE uses a highly detailed and semiscripted curriculum that makes interventionist training potentially feasible and practical.4,6 In our experience, social worker interventionist training is readily completed over a period of 4 weeks.
Change in BD depressive symptoms as measured by a mean sample total score on the MADRS appeared to optimize at about the halfway point in the study (Figure 2), suggesting that symptom improvement may take some time to occur once treatment adherence is established. It is possible that the robust improvement in depressive symptom severity overall could have been driving some of the other outcome changes, such as improvement in general and social and occupational functioning.
The majority (57%) of patients had multiple barriers to medication adherence identified at screening evaluation. Patients improved their knowledge about BD, management of their medication routines, and readiness to change for substance use patterns. Underscoring the common occurrence of substance use disorders among people with BD, most (86.7%) individuals in this sample were assigned to receive the CAE module that addresses substance use based on their adherence barrier screening assessment. While reported readiness to change substance use patterns did improve during the course of the trial, self-reported substance use behaviors as assessed by the AUDIT and DAST-10 did not significantly change. The question of whether actual substance use behaviors could be changed with CAE likely needs to be assessed in larger studies, ideally of longer duration and with more objective measures of substance use. We also did not find significant improvement in communication with providers. Perhaps involving prescribing providers might be necessary to help yield change in this domain of clinical alliance.
The LAI used in this study, once-monthly aripiprazole, was generally well tolerated, with the most common side effects being sedation and injection site pain. Both of these side effects tended to diminish over time. While there were no significant group mean changes on extrapyramidal symptoms, one individual had to stop the study drug due to tremor, which resolved with LAI discontinuation. There were no group mean changes on metabolic outcomes, but approximately 20% of individuals had weight gain > 7% of baseline body mass index. Weight gain is a common side effect of second-generation antipsychotic drugs, although aripiprazole may be associated with less weight gain in relation to some other antipsychotic compounds.50 In spite of side effects for some individuals and their existing history of poor adherence, two-thirds of individuals planned to continue LAI post study.
This study had a number of limitations including small sample, noncontrolled design, and single-site setting. An additional limitation is that adherence was based on self-report, which has potential to undercount missed medication. The improvement in adherence behaviors immediately after screen (and before CAE was administered) may possibly reflect a Hawthorne effect, which could have obscured improvement in CAE-related change. However, in the broader BD adherence literature, missing ≤ 20% of prescribed psychotropic medication is generally considered “good” adherence,2 suggesting that the sample 24-week means of missed drug (TRQ score of 15.5% last week and 17.6% last month) is of clinical relevance. Other strengths of the study include the diverse sample and that individuals with known poor adherence were specifically enrolled, a group that is willing to acknowledge problem adherence and for whom adherence promotion efforts may be particularly impactful. Overall, findings suggest that a personalized intervention to address adherence barriers combined with LAI can improve multiple recovery outcomes in high-risk individuals with BD. Controlled and larger studies, with the addition of an objective measure of adherence behavior, such as automated pill counts or serum mood stabilizer levels, are needed to confirm these preliminary findings.
Submitted: December 4, 2020; accepted February 19, 2021.
Published online: September 16, 2021.
Potential conflicts of interest: Dr Sajatovic has received grant support from Nuromate, Otsuka, Alkermes, International Society for Bipolar Disorders, National Institutes of Health, Centers for Disease Control and Prevention, and Patient-Centered Outcomes Research Institute; has been a consultant to Alkermes, Otsuka, Janssen, Neurocrine, Bracket, Health Analytics, and Frontline Medical Communications; has received publication royalties from Springer Press, Johns Hopkins University Press, Oxford Press, and UpToDate; and has received compensation for preparation of CME activities from the American Physician’s Institute, MCM Education, CMEology, Potomac Center for Medical Education, Global Medical Education, Creative Educational Concepts, and Psychopharmacology Institute. Dr Ramirez has served as a speaker for Bristol-Myers Squibb, Merck, Novartis, and Janssen in the past and currently serves as a speaker for Otsuka and Sunovion and on advisory boards for Teva and Vanta. Dr McNamara has received research funding from Shire, Sunovion, Otsuka, Roche, Genetech, Takeda/Lundbeck, Duke Clinical Research Institute, and Hartwell Foundation and has served as a consultant to Shire and Roche. Drs Levin and Briggs; Mss Cassidy, Fuentes-Casiano, and Wilson; and Mr Appling report no conflicts of interest related to the subject of this article.
Funding/support: Funding for this project was provided by an investigator-initiated grant from Otsuka. Support was also provided by the Clinical and Translational Science Collaborative (Dahms Clinical Research Unit) NIH grant number UL1 RR024989.
Role of the sponsor: The sponsor was not involved in the conduct of the study, collection, management, analysis, interpretation, or report preparation. The sponsor was provided an opportunity to review the manuscript and provide input. Publication was not contingent on sponsor approval.
Clinical Points
- Poor medication adherence is widely prevalent in people with bipolar disorder and is often associated with negative outcomes.
- Clinicians should consider barriers to adherence that are person specific and potentially modifiable.
- Combining long-acting injectable antipsychotic medication with a personalized barrier-focused behavioral approach can improve adherence, symptoms, and functioning in people with bipolar disorder.
References (50)

- Jawad I, Watson S, Haddad PM, et al. Medication nonadherence in bipolar disorder: a narrative review. Ther Adv Psychopharmacol. 2018;8(12):349–363. PubMed CrossRef
- Levin JB, Krivenko A, Howland M, et al. Medication adherence in patients with bipolar disorder: a comprehensive review. CNS Drugs. 2016;30(9):819–835. PubMed CrossRef
- Hartung D, Low A, Jindai K, et al. Interventions to improve pharmacological adherence among adults with psychotic spectrum disorders and bipolar disorder: a systematic review. Psychosomatics. 2017;58(2):101–112. PubMed CrossRef
- Sajatovic M, Tatsuoka C, Cassidy KA, et al. A 6-month, prospective, randomized controlled trial of customized adherence enhancement versus bipolar-specific educational control in poorly adherent individuals with bipolar disorder. J Clin Psychiatry. 2018;79(6):17m12036. PubMed CrossRef
- Levin JB, Tatsuoka C, Cassidy KA, et al. Trajectories of medication attitudes and adherence behavior change in non-adherent bipolar patients. Compr Psychiatry. 2015;58:29–36. PubMed
- Sajatovic M, Levin J, Ramirez LF, et al. Prospective trial of customized adherence enhancement plus long-acting injectable antipsychotic medication in homeless or recently homeless individuals with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2013;74(12):1249–1255. PubMed CrossRef
- Sajatovic M, Levin J, Tatsuoka C, et al. Six-month outcomes of customized adherence enhancement (CAE) therapy in bipolar disorder. Bipolar Disord. 2012;14(3):291–300. PubMed CrossRef
- Sajatovic M, Levin J, Tatsuoka C, et al. Customized adherence enhancement for individuals with bipolar disorder receiving antipsychotic therapy. Psychiatr Serv. 2012;63(2):176–178. PubMed CrossRef
- Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495–553. PubMed CrossRef
- Llorca PM, Abbar M, Courtet P, et al. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13(1):340. PubMed CrossRef
- Bauer M, Glenn T, Alda M, et al. Drug treatment patterns in bipolar disorder: analysis of long-term self-reported data. Int J Bipolar Disord. 2013;1(1):5. PubMed CrossRef
- Zeber JE, Miller AL, Copeland LA, et al. Medication adherence, ethnicity, and the influence of multiple psychosocial and financial barriers. Adm Policy Ment Health. 2011;38(2):86–95. PubMed CrossRef
- Sajatovic M, Ramirez LF, Fuentes-Casiano E, et al. A 6-month prospective trial of a personalized behavioral intervention + long-acting injectable antipsychotic in individuals with schizophrenia at risk of treatment nonadherence and homelessness. J Clin Psychopharmacol. 2017;37(6):702–707. PubMed CrossRef
- Peet M, Harvey NS. Lithium maintenance, 1: a standard education programme for patients. Br J Psychiatry. 1991;158(2):197–200. PubMed CrossRef
- Scott J, Pope M. Nonadherence with mood stabilizers: prevalence and predictors. J Clin Psychiatry. 2002;63(5):384–390. PubMed CrossRef
- Sheehan DV, Lecubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33, quiz 34–57. PubMed
- Overall JA, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799–812. CrossRef
- Muneer A. The treatment of adult bipolar disorder with aripiprazole: a systematic review. Cureus. 2016;8(4):e562. PubMed CrossRef
- Torres-Llenza V, Lakshmin P, Lieberman DZ. Spotlight on once-monthly long-acting injectable aripiprazole and its potential as maintenance treatment for bipolar I disorder in adult patients. Neuropsychiatr Dis Treat. 2018;14:285–292. PubMed CrossRef
- Yan T, Greene M, Chang E, et al. Medication adherence and discontinuation of aripiprazole once-monthly 400 mg (AOM 400) versus oral antipsychotics in patients with schizophrenia or bipolar i disorder: a real-world study using US claims data. Adv Ther. 2018;35(10):1612–1625. PubMed CrossRef
- Bauer MS. Supporting collaborative practice management: The Life Goals Program. In: Johnson SL, Leahy R, eds. Psychological Treatment of Bipolar Disorder. New York: Guilford Press; 2004:203–225.
- Bauer MS, McBride L, Williford WO, et al; Cooperative Studies Program 430 Study Team. Collaborative care for bipolar disorder, part I: intervention and implementation in a randomized effectiveness trial. Psychiatr Serv. 2006a;57(7):927–936. PubMed CrossRef
- Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62(9):996–1004. PubMed CrossRef
- Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62(9):996–1004. PubMed CrossRef
- Sajatovic M, Davies M, Bauer MS, et al. Attitudes regarding the collaborative practice model and treatment adherence among individuals with bipolar disorder. Compr Psychiatry. 2005;46(4):272–277. PubMed CrossRef
- Ziedonis DM, Smelson D, Rosenthal RN, et al. Improving the care of individuals with schizophrenia and substance use disorders: consensus recommendations. J Psychiatr Pract. 2005;11(5):315–339. PubMed CrossRef
- Sajatovic M, Velligan DI, Weiden PJ, et al. Measurement of psychiatric treatment adherence. J Psychosom Res. 2010;69(6):591–599. PubMed CrossRef
- Sajatovic M, Levin JB, Sams J, et al. Symptom severity, self-reported adherence, and electronic pill monitoring in poorly adherent patients with bipolar disorder. Bipolar Disord. 2015;17(6):653–661. PubMed CrossRef
- Awad AG. Subjective response to neuroleptics in schizophrenia. Schizophr Bull. 1993;19(3):609–618. PubMed CrossRef
- Scott J. Predicting medication non-adherence in severe affective disorders. Acta Neuropsychiatr. 2000;12(3):128–130. PubMed CrossRef
- Bilderbeck AC, Atkinson LZ, McMahon HC, et al. Psychoeducation and online mood tracking for patients with bipolar disorder: a randomised controlled trial. J Affect Disord. 2016;205:245–251. PubMed CrossRef
- Verplanken B, Orbell S. Reflections on past behavior: a self-report index of habit strength. J Appl Soc Psychol. 2003;33(6):1313–1330. CrossRef
- Bultman DC, Svarstad BL. Effects of physician communication style on client medication beliefs and adherence with antidepressant treatment. Patient Educ Couns. 2000;40(2):173–185. PubMed CrossRef
- Miller WR, Scott TJ. Assessing drinkers’ motivation for change: the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES). Psychol Addict Behav. 1996;10(2):81–89. CrossRef
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand suppl. 1970;212(S212):11–19. PubMed CrossRef
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672–676. PubMed CrossRef
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Washington, DC: US Department of Health, Education, and Welfare; 1976.
- Chouinard G. The Extrapyramidal Symptoms Rating Scale. Can J Neurol Sci. 1980;7:233–244.
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(Nov):429–435. PubMed CrossRef
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. PubMed CrossRef
- Babor TF, Higgins-Biddle JC, Saunders JB, et al. The Alcohol Use Disorder Identification Test: Guidelines for Use in Primary Care. 2nd ed. World Health Organization; 2001.
- Skinner HA. The Drug Abuse Screening Test. Addict Behav. 1982;7(4):363–371. PubMed CrossRef
- Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323–329. PubMed CrossRef
- Spitzer R, Gibbon M, Williams J. Global Assessment of Functioning (GAF) Scale. Baltimore, MD: Williams and Wilkins; 1996.
- Howieson DB, Loring DW, Lezak MD. Neuropsychological Assessment. 4th ed. New York, New York: Oxford University Press; 2004.
- Lezak MD. Neuropsychological Assessment. 5th ed. New York, New York: Oxford University Press; 2012.
- García S, Martínez-Cengotitabengoa M, López-Zurbano S, et al. Adherence to antipsychotic medication in bipolar disorder and schizophrenic patients: a systematic review. J Clin Psychopharmacol. 2016;36(4):355–371. PubMed CrossRef
- Leclerc E, Mansur RB, Brietzke E. Determinants of adherence to treatment in bipolar disorder: a comprehensive review. J Affect Disord. 2013;149(1-3):247–252. PubMed CrossRef
- Johnson FR, Ozdemir S, Manjunath R, et al. Factors that affect adherence to bipolar disorder treatments: a stated-preference approach. Med Care. 2007;45(6):545–552. PubMed CrossRef
- Bak M, Fransen A, Janssen J, et al. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9(4):e94112. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top