ABSTRACT
Objective: To assess the long-term efficacy and safety of aripiprazole as an augmentation strategy for major depressive disorder (MDD).
Data Sources: Ovid MEDLINE, PsycInfo, and Embase databases were systematically searched for clinical studies of adult patients with MDD on long-term aripiprazole augmentation.
Study Selection and Data Extraction: Long-term follow-up was defined as ≥ 6 months. Primary outcome was remission from depression. Secondary outcome was incidence of adverse effects.
Results: Four open-label studies were included in this review. Random effects meta-analysis of 3 studies (n = 2,117) revealed a weighted average remission proportion of 0.33 (0.16–0.51), showing trend of improved response with duration of treatment. Three studies (n = 2,231) provided adverse effects data. Medically significant weight gain (25%–28% participants) was higher in studies with patients receiving doses ≥ 5 mg and lower (3.5%) in a study using doses < 5 mg. Akathisia (15%–16%), insomnia (12%–17%), somnolence (14%), and fatigue (18%) were common adverse effects. Tardive dyskinesia risk was low (< 1%) at 1-year follow-up. All included studies were open-label, noncontrolled studies, with longest follow-up of 52 weeks, limiting efficacy and safety conclusions.
Conclusions: Aripiprazole augmentation may be an effective long-term strategy for treatment of refractory MDD. Lower maintenance doses (2–5 mg) of aripiprazole may be effective and have fewer side effects compared to larger doses (> 5 mg–10 mg).
Prim Care Companion CNS Disord 2021;23(4):20r02799
To cite: Seshadri A, Wermers ME, Habermann TJ, et al. Long-term efficacy and tolerability of adjunctive aripiprazole for major depressive disorder: systematic review and meta-analysis. Prim Care Companion CNS Disord. 2021;23(4):20r02799.
To share: https://doi.org/10.4088/PCC.20r02799
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Psychology, Mayo Clinic Health System, Austin, Minnesota
bDepartment of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota
cUniversity of Minnesota, College of Pharmacy, Minneapolis, Minnesota
dMedical College of Wisconsin, Wauwatosa, Wisconsin
eDepartment of Pharmacy, Mayo Clinic Health System, Austin, Minnesota
*Corresponding author: Ashok Seshadri, MD, Department of Psychiatry and Psychology, Mayo Clinic, 1000 1st Drive NW, Austin, Minnesota, 55912 ([email protected]).
Major depressive disorder (MDD) is a leading cause of disability worldwide.1 More than 4% of the world’s population suffers from depression. Only 50% of patients with MDD respond to the first trial of an antidepressant, and 36.8% of patients achieve remission after a first course of antidepressant treatment.1,2 Patients with MDD who respond partially to an adequate trial of antidepressant often need an augmentation agent to further optimize the response.3,4 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study was a pragmatic effectiveness trial wherein subjects with depression not achieving remission from citalopram were sequentially switched or augmented with alternative medication strategies. This study demonstrated the effectiveness of a flexible treatment approach that included augmentation strategies with medications such as mirtazapine, buspirone, bupropion, lithium, triiodothyronine, and nortriptyline if patients did not achieve adequate response to treatment.5 Atypical antipsychotic medication augmentation strategies using olanzapine, quetiapine, and aripiprazole have also been shown to result in improved depressive outcomes in randomized controlled studies of 6- to 12-week duration.6–11 Olanzapine and quetiapine are, however, associated with significant risk of weight gain and sedation, making them less desirable for long-term maintenance therapy.6,8
Aripiprazole is an atypical antipsychotic medication that was US Food and Drug–approved as an adjunct for MDD in 2007.12 Aripiprazole is unique in its mechanism of action as a partial agonist at dopamine D2, D3, and serotonin 5-HT1A receptors and an antagonist at 5-HT2A receptors.13 Randomized controlled trials (RCTs) of aripiprazole investigating its efficacy as an augmentation agent for depression for short-term (6 to 8 weeks) duration showed higher response rates compared to control.9–11,14,15 Common side effects reported from these studies9–11,14,15 included akathisia, fatigue, and weight gain.
There is a scarcity of data regarding the safety and efficacy of aripiprazole as a maintenance augmentation strategy for MDD. The objective of this review is to systematically synthesize the literature to determine long-term efficacy and safety of aripiprazole as a maintenance augmenting agent for MDD.
METHODS
A systematic search of Ovid MEDLINE, Embase, and PsycInfo databases from inception of the databases to May 23, 2019, was conducted by a professional librarian. We searched for studies with participants (P) with MDD treated with intervention (I), and aripiprazole augmentation of an antidepressant compared to control or placebo (C). Our outcomes of interest (O) were long-term remission/response and long-term adverse effects. We restricted our search to RCTs, open-label trials, and observational studies published in the English language. Long-term follow-up was defined as study duration ≥ 6 months. Our inclusion criteria for participants were adults (≥ 18 years of age) with MDD, with inadequate response to an antidepressant after at least 6 weeks of treatment, and treated with aripiprazole compared against any control condition.
Study Selection and Data Extraction
The online systematic review tool Endnote was used for title and abstract screening and full-text review.16 Title and abstract screening were done in duplicate by 2 independent reviewers (M.L.W. and A.S.). Conflicts were resolved by discussion. Full-text review was completed in duplicate from studies meeting inclusion criteria by 2 authors (M.L.W. and A.S.). Any disagreements were resolved by consensus. Treatment response data were extracted using remission rates from selected studies. We extracted side effects data reported from selected studies.
Quality Assessment/Risk of Bias
Quality assessment was carried out in duplicate by 2 study authors (A.S. and B.S.) using a modified version of the Newcastle-Ottawa Scale (NOS) adapted for cross-sectional studies.17 Conflicts were resolved by discussion and consensus. The modified NOS scale uses a points system (1 point or 0 points) to evaluate included studies assessing sample representativeness and size, comparability between responders and non-responders, ascertainment of depression outcome, and thoroughness of outcome reporting (Supplementary Table 1). Quantitative tests to assess publication bias were not performed due to the limited number of studies.18
Meta-analytic Techniques Consideration
Treatment response data were extracted using reported rates of remission from selected studies. Meta-analysis of proportions was performed using a random effects model to synthesize the weighted average proportions of remission from the selected studies, weighted by using the inverse variance method.19 RStudio version 3.5.1 software was used to conduct the meta-analysis.20 Heterogeneity was assessed using Cochran’s Q statistic with P value < .10 on the Cochran’s Q test used as a cutoff to attribute heterogeneity to between study factors rather than by chance.21 The I2 statistic was used to assess the contribution of between-study heterogeneity to the overall estimate of heterogeneity. We assigned cutoffs of 25%, 50%, and 75% for small, moderate, and large levels of heterogeneity, respectively, between studies.21
RESULTS
Our search strategy yielded 810 articles. After deduplication, 609 records were screened by title and abstract, from which 18 studies were found to be eligible for full-text review (Supplementary Figure 1). Of the full-text articles assessed for eligibility, only 4 studies provided long-term data (≥ 6 months) of efficacy or incidence of adverse effects.22–25 Two studies were open-label aripiprazole augmentation trials.24,25 One study was a prospective observational study of 52-week duration,22 and 1 study used a RCT design for the acute phase followed by a 24-week open-phase follow-up period.23 The studies were done in the United States (2),23,25 Italy (1),24 and Japan (1).22 Across the 4 studies, a total of 2,632 participants received aripiprazole augmentation therapy.
Characteristics of Included Studies
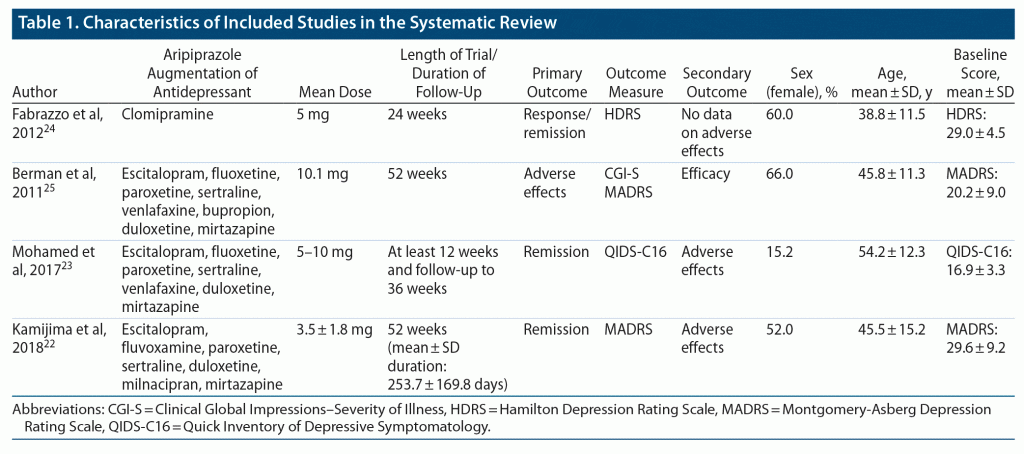
Berman et al25 conducted an open-label augmentation study of aripiprazole to assess long-term safety with a 52-week follow-up that included 1,002 adult patients who received adjunctive aripiprazole to antidepressant medications (escitalopram, fluoxetine, paroxetine, sertraline, venlafaxine, bupropion, duloxetine, mirtazapine) with the target aripiprazole dose of 10 mg/d. Fabrazzo et al24 completed an open-label augmentation study of aripiprazole with clomipramine (100 mg–300 mg) to assess efficacy in participants with MDD not responsive to clomipramine alone after an ineffective previous trial of SSRI medication. They used a fixed dose of aripiprazole 5 mg/d with a 24-week follow-up period.24 The VA Augmentation and Switching Treatments for Improving Depression Outcomes (VAST-D)23 was a multisite RCT with participants having suboptimal response to a course of SSRI, SNRI, or mirtazapine. The study compared participants randomized to aripiprazole augmentation of antidepressant medication (citalopram, escitalopram, fluoxetine, paroxetine, sertraline, venlafaxine, duloxetine, or mirtazapine) to participants with bupropion augmentation of antidepressant and participants switched to bupropion monotherapy during the acute treatment phase of 12 weeks with subsequent 24-week open-label follow-up if they showed adequate benefit and tolerated medication therapy.23 Kamijima et al22 conducted a prospective multicenter, observational study of 1,103 participants with MDD, followed for 52 weeks of aripiprazole augmentation of antidepressant therapy (escitalopram, fluvoxamine, paroxetine, sertraline, duloxetine, mirtazapine, or milnacipran) to assess effectiveness and safety of aripiprazole augmentation in real-world clinical practice settings. Table 1 provides a summary of study characteristics of the 4 studies included in this systematic review.
Efficacy of Long-term Aripiprazole Augmentation
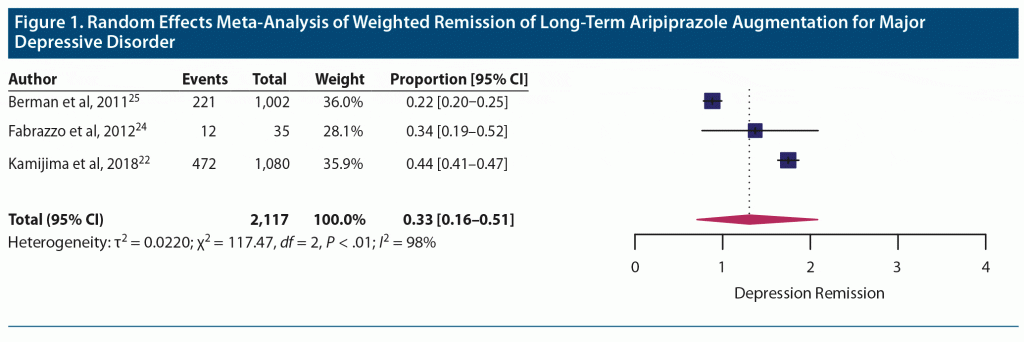
Efficacy. Random effects meta-analysis of 3 long-term aripiprazole augmentation studies showed a weighted remission proportion of 0.33 (0.16–0.51), suggesting a weighted average of one-third of participants achieving depression remission with long-term treatment (Figure 1). Heterogeneity was high between included studies (Q = 1,172; df = ; P < .01) with high between-study heterogeneity (I2 = 98%). Rates of remission showed a trend toward improved remission rates with lower average doses among the 3 studies.
Berman et al,25 with a mean dose of 10.1 mg/d, provided efficacy data using the Clinical Global Impressions-Severity of illness (CGI-S) rating scale and reporting 22% of participants meeting criteria for “normal” or “borderline ill.” Fabrazzo et al24 reported remission rates of 34% after 24 weeks of combined treatment with clomipramine (mean dose = 181 mg/d, SD = 56.9) and a fixed augmentation dose of aripiprazole (5 mg/d). They observed a trend of increasing response (57% at week 8 to 91% at week 24) and remission (0 at week 8 to 34% at week 24) with duration of treatment.24 Kamijima et al,22 with a mean ± SD dose of 3.5 ± 1.8 mg/d, reported a similar trend of statistically significant improvement in mean depression scores measured using the Montgomery-Asberg Depression Rating Scale (MADRS) from baseline to each follow-up time point of the study (months 1, 3, 6, and 12). Remission was achieved by 34.5% of participants at 6 months and 43.3% of participants at 12 months. In this study, there were no statistically significant differences based on antidepressants that were being augmented. Logistic regression analysis revealed mild to moderate disease severity, MADRS scores < 33 (P = .013), and shorter duration of depression (< 176 days, P < .001) were associated with significantly greater response to aripiprazole augmentation.22
Safety/Tolerability of Long-term Aripiprazole Augmentation
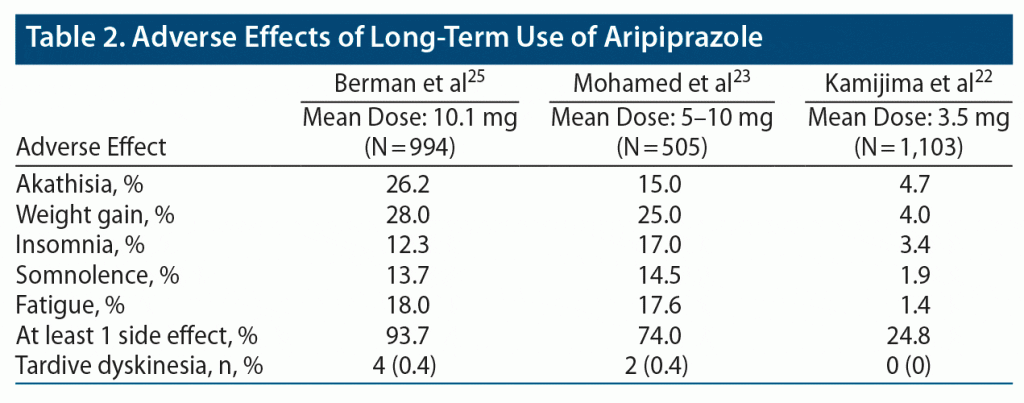
Three studies provided data of adverse effects noted in their studies22,23,25 (Table 2), while 1 study reported no significant adverse effects including weight changes with aripiprazole augmentation.24 The incidence of at least 1 treatment-emergent side effect appeared to show a dose-dependent relationship based on reported side effects from these studies. The study22 with the lowest mean dose of 3.5 mg daily had the lowest rate of at least 1 side effect with 24.8%, while the study25 with the highest mean average dose of 10.1 mg had the highest rate at 93.7%. The study with a mean dose between 5 mg and 10 mg reported 74% of participants had at least 2 side effect.23 The most common adverse effects were akathisia, weight gain, insomnia, somnolence, and fatigue, with incidence of akathisia and weight gain greatest in the study using doses > 10 mg. Berman et al25 reported medically significant weight gain, defined as ≥ 7% body weight, in 28% of subjects, with most weight gain occurring within the first 26 to 32 weeks of treatment, while Mohamed et al23 reported a 25.2% incidence of medically significant weight gain. By contrast, the study22 using low doses < 3.5 mg/d reported only 4.4% incidence of weight gain. Insomnia and somnolence occurred in 12%–17% of participants receiving doses above 5 mg. Tardive dyskinesia risk was low at 1-year follow-up, occurring in < 1% in the 2 studies23,25 using doses > 5 mg, and 0% in the study22 using a mean dose of 3.5 mg. There was no significant change in mean fasting cholesterol or glucose levels compared to baseline in 1 study.25 Prolactin elevations occurred occasionally during serial monitoring, but did not occur in a predictable fashion. Overall, mean serum prolactin levels were lower at week 52 compared to baseline.25
Quality of Included Studies
All the included long-term studies used an open-label design. Two22,25 of the 4 included studies contained large samples followed for 52 weeks, drawn from multiple clinical sites. Two studies22,23 provided comparative data between treatment responders and nonresponders. All studies used validated outcome measures. Three22,23,25 of 4 studies provided descriptive statistics of event rates for primary outcome measures. Based on our modified criteria, 1 study scored 5 points,22 1 study scored 4 points,25 1 study scored 3 points,23 and 1 study scored 1 point.24
DISCUSSION
Implications for Practice
In this systematic review, we identified 4 studies that evaluated the long-term use of aripiprazole augmentation with commonly used antidepressants including SSRIs, SNRIs, TCA (clomipramine), bupropion, and mirtazapine for efficacy and safety. Findings from 3 long-term studies showed that aripiprazole augmentation of antidepressant medications was associated with significant improvement in depression outcome measures irrespective of concomitant antidepressant medication starting early in treatment with continued improvement through month 12. A trend of a gradually increasing proportion of participants achieving remission from depression was observed with continued treatment to month 12.22,24 Two22,24 of these studies used aripiprazole doses ≤ 5 mg for augmentation. Adverse effects data suggest that doses of aripiprazole > 5 mg are associated with greater side effect burden including occurrence of medically significant weight gain in 25% to 28% of patients, as well as akathisia (15%–16%), insomnia (12%–17%), somnolence (14%), and fatigue (18%). Risk of tardive dyskinesia was low (< 1%) based on these studies.
Recurrent MDD is treated with long-term maintenance of medication treatment to reduce the risk of relapse.26 These long-term studies do not provide data to recommend optimal duration of maintenance therapy. However, relative safety of low-dose augmentation and suggestion of increasing benefit from longer treatment duration suggest aripiprazole could be a safe long-term maintenance strategy for up to 52 weeks. However, it must be noted that the studies reporting treatment response data included in this review were open-label studies with no control groups, making firm conclusions on efficacy difficult. Additionally, side effects such as tardive dyskinesia could emerge many years after treatment. Although there was no suggestion of significant changes in fasting blood sugars or cholesterol, medically significant weight gain occurred in 25% of participants in 2 studies. The metabolic impact of weight gain may manifest over a longer timeframe than was possible to evaluate with a 1-year follow-up period.
Implications for Future Research
Future studies using a RCT design with a control group will provide a greater ability to assess treatment efficacy and adverse effects more accurately. Naturalistic follow-up studies of > 1-year duration are needed to determine long-term effectiveness and safety of maintenance aripiprazole augmentation for MDD. While the studies reviewed here suggest increasing rates of remission with duration of treatment, it is unclear how long the benefits may be sustained. It is unclear if future relapses or recurrence may be responsive to dose escalation strategies and what the side effect burden of that strategy may be. Some adverse effects such as tardive dyskinesia may develop many years after treatment. Additionally, studies comparing the efficacy of aripiprazole augmentation at different doses as well as with other augmentation strategies like lithium, lamotrigine, and other antipsychotic medication augmentation strategies could help inform treatment strategies that balance the benefits versus relative risks of each treatment approach.
Limitations
One of the limitations of our review was the small number of studies identified with long-term follow-up that ranged from 24 to 52 weeks. The observational, open-label nature of included studies without a control group precludes making conclusions on the efficacy and tolerability of aripiprazole as an augmentation strategy. Although longer durations of follow-up in the included studies are greater than most clinical trials, the recurring and relapsing nature of MDD demands treatment duration with medications much greater than 1 year. Some of the statistical significance seen in the included studies could be due to the large sample sizes employed in the study designs.
CONCLUSIONS
This systematic review of 4 open-label long-term follow-up studies of 24 to 52 weeks of aripiprazole augmentation of antidepressants for MDD suggest that aripiprazole may be a helpful maintenance strategy with lower doses (< 5 mg) being more efficacious while causing fewer side effects compared to larger doses (5 mg–10 mg). There is suggestion of increasing treatment benefits with increased duration of treatment. Most common adverse effects were akathisia, weight gain, insomnia, somnolence, and fatigue.
Clinical Points
- Aripiprazole augmentation may be an effective long-term strategy for treatment of refractory depression.
- Lower maintenance doses (< 5 mg) of aripiprazole may be effective and have fewer side effects compared to larger doses.
Submitted: August 19, 2020; accepted November 17, 2020.
Published online: June 24, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Supplementary material: See accompanying pages.
References (26)

- Depression and other Common Mental Disorders: Global Health Estimates. World Health Organization. 2017. https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf
- Rush AJ, Trivedi MH, Wisniewski SR, et al; STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231–1242. PubMed CrossRef
- Depression in Adults: Recognition and Management. NICE Clinical Guidelines, No. 90. Updated 2009. Accessed 2020. https://www.ncbi.nlm.nih.gov/books/NBK553259/.
- Gelenberg AJ, Freeman MP, Markowitz JC, et al; Work Group on Major Depressive Disorder. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- Corya SA, Williamson D, Sanger TM, et al. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, fluoxetine, and venlafaxine in treatment-resistant depression. Depress Anxiety. 2006;23(6):364–372. PubMed CrossRef
- Thase ME, Corya SA, Osuntokun O, et al. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry. 2007;68(2):224–236. PubMed CrossRef
- Bauer M, Pretorius HW, Constant EL, et al. Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: results of a randomized, placebo-controlled, double-blind study. J Clin Psychiatry. 2009;70(4):540–549. PubMed CrossRef
- Berman RM, Fava M, Thase ME, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14(4):197–206. PubMed CrossRef
- Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404–2412. PubMed CrossRef
- Kamijima K, Higuchi T, Ishigooka J, et al; ADMIRE Study Group. Aripiprazole augmentation to antidepressant therapy in Japanese patients with major depressive disorder: a randomized, double-blind, placebo-controlled study (ADMIRE study). J Affect Disord. 2013;151(3):899–905. PubMed CrossRef
- Abilify (aripiprazole) [package insert]. Rockville, MD: Otsuka America Pharmaceutical Inc; 2020.
- Shapiro DA, Renock S, Arrington E, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–1411. PubMed CrossRef
- Berman RM, Marcus RN, Swanink R, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(6):843–853. PubMed CrossRef
- Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28(2):156–165. PubMed CrossRef
- EndNote X9. Clarivate. Published 2019. Accessed August 14, 2020. https://endnote.com/
- Wells GASB, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital. Accessed July 09, 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–1129. PubMed CrossRef
- Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. PubMed CrossRef
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC. Published 2020. Accessed 2020. http://www.rstudio.com/
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. PubMed CrossRef
- Kamijima K, Yasuda M, Yamamura K, et al. Real-world effectiveness and safety of aripiprazole augmentation therapy in patients with major depressive disorder. Curr Med Res Opin. 2018;34(12):2105–2112. PubMed CrossRef
- Mohamed S, Johnson GR, Chen P, et al; and the VAST-D Investigators. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132–145. PubMed CrossRef
- Fabrazzo M, Perris F, Monteleone P, et al. Aripiprazole augmentation strategy in clomipramine-resistant depressive patients: an open preliminary study. Eur Neuropsychopharmacol. 2012;22(2):132–136. PubMed CrossRef
- Berman RM, Thase ME, Trivedi MH, et al. Long-term safety and tolerability of open-label aripiprazole augmentation of antidepressant therapy in major depressive disorder. Neuropsychiatr Dis Treat. 2011;7:303–312. PubMed CrossRef
- Glue P, Donovan MR, Kolluri S, et al. Meta-analysis of relapse prevention antidepressant trials in depressive disorders. Aust N Z J Psychiatry. 2010;44(8):697–705. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top