Objective: Community-dwelling older adults with neurocognitive disorders experience high risk of and often suffer severe consequences from medication nonadherence. Due to the important role of informal caregivers in the care of patients with neurocognitive disorders, medication management involves both patients and families. A formal diagnosis of a neurocognitive disorder may improve both provider-patient and provider-family communications and resulting regimen adherence, yet many with signs of neurocognitive disorders remain undiagnosed. The goal of this study was to examine the differences in medication management behaviors for family caregivers of mildly impaired older adults with or without a formal neurocognitive disorder diagnosis.
Method: The study included 112 women who provided at least 2 forms of medication assistance for a mildly cognitively impaired older adult with (n = 38, 34%) or without (n = 75, 66%) a reported neurocognitive disorder diagnosis and who completed online self-assessments of medication adherence and self-efficacy for medication management from May 2012 to May 2013. Cases were selected for analyses based on analog Clinical Dementia Rating scores between 0.5 and 1, indicating mild cognitive impairment in the older adult.
Results: Compared to families unaware of a neurocognitive disorder diagnosis, caregivers reporting knowledge of a neurocognitive disorder diagnosis in their older family member endorsed higher medication management self-efficacy and increased levels of adherence-related behaviors. Step-wise logistic regression analyses demonstrated statistical significance in using these adherence and self-efficacy variables to differentiate between the presence or absence of a known neurocognitive disorder diagnosis (N = 112, χ26 = 22.84, P < .05).
Conclusions: A formally charted and communicated neurocognitive disorder diagnosis is associated with improved medication management behaviors and medication-related self-efficacy in neurocognitive disorder family caregivers.
Objective: Community-dwelling older adults with neurocognitive disorders experience high risk of and often suffer severe consequences from medication nonadherence. Due to the important role of informal caregivers in the care of patients with neurocognitive disorders, medication management involves both patients and families. A formal diagnosis of a neurocognitive disorder may improve both provider-patient and provider-family communications and resulting regimen adherence, yet many with signs of neurocognitive disorders remain undiagnosed. The goal of this study was to examine the differences in medication management behaviors for family caregivers of mildly impaired older adults with or without a formal neurocognitive disorder diagnosis.
Method: The study included 112 women who provided at least 2 forms of medication assistance for a mildly cognitively impaired older adult with (n = 38, 34%) or without (n = 75, 66%) a reported neurocognitive disorder diagnosis and who completed online self-assessments of medication adherence and self-efficacy for medication management from May 2012 to May 2013. Cases were selected for analyses based on analog Clinical Dementia Rating scores between 0.5 and 1, indicating mild cognitive impairment in the older adult.
Results: Compared to families unaware of a neurocognitive disorder diagnosis, caregivers reporting knowledge of a neurocognitive disorder diagnosis in their older family member endorsed higher medication management self-efficacy and increased levels of adherence-related behaviors. Step-wise logistic regression analyses demonstrated statistical significance in using these adherence and self-efficacy variables to differentiate between the presence or absence of a known neurocognitive disorder diagnosis (N = 112, χ26 = 22.84, P < .05).
Conclusions: A formally charted and communicated neurocognitive disorder diagnosis is associated with improved medication management behaviors and medication-related self-efficacy in neurocognitive disorder family caregivers.
Prim Care Companion CNS Disord 2014;16(6):doi:10.4088/PCC.14m01686
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: June 4, 2014; accepted July 30, 2014.
Published online: December 4, 2014.
Corresponding author: Nika R. George, MA, One University Blvd, Stadler Hall Room 325B, St Louis, MO 63121 ([email protected]).
Medication nonadherence is a pressing behavioral health issue for older adults living in the community.1 On average, individuals aged 65 years and older are diagnosed with 3 to 5 chronic health conditions managed with an estimated 5 medications.2 Accordingly, older adults have more opportunities for nonadherence than other segments of the population.3 Estimates vary slightly, but most projections indicate that over 50% of older adults in the community do not follow regimens as prescribed4–6 and have poorer physical health outcomes and more preventable injuries than those who follow regimen instructions.7 These injuries are made more likely due to age-related physical vulnerabilities.8 Older adults who take medications as instructed experience fewer disabilities and enjoy improved or stabilized chronic health conditions,4,7 while those who do not may experience frailty, ambulatory concerns, disability related to exacerbated health conditions, objective decreases in positive indicators of health, and shorter life expectancies.4,7,9,10
Nonadherence and resulting adverse drug events can also result in high financial cost from increased emergency department visits, elongated hospital stays, and added physician visits or medical testing.11–13 Approximately 30% of all hospital visits and 11.4% of emergency room admissions in older adults are linked to medication nonadherence.14,15 On a macroscopic scale, medication nonadherence results in costs paid by the federal government through taxpayer funds.16,17 Improved medication adherence, however, could very likely reduce these economic costs.
Older adults with Alzheimer’s disease or other neurocognitive disorders are especially likely to become medication nonadherent.18 Lower scores on brief cognitive measures have been shown to predict nonadherence in older adults with multiple chronic health conditions.19,20 When compared to older adults who do not exhibit cognitive decline, individuals with a neurocognitive disorder demonstrate broadly worse levels of adherence.14,21,22 In studies examining rates of adverse drug events related to nonadherence in older adults, post hoc analysis reveals cognitive limitations to be present in the majority of adverse drug event cases.23 In addition, older adults with neurocognitive disorders are often prescribed increased numbers of medications, including antipsychotics, which increase opportunities for regimen deviations.15 Overall, nonadherence in persons with neurocognitive disorders causes more accidental deaths than fires and wandering14; these individuals are also more susceptible to episodes of delirium brought about by medication nonadherence,24,25 with increased risk for accelerated cognitive decline, multiple rehospitalizations, and death.24,25 Approximately 5.2 million Americans currently have a dementing illness, and, by 2050, the number of senior citizens with incurable neurocognitive disorders will increase to approximately 16 million.26
Persons with neurocognitive disorders are not the only individuals directly impacted by these medication concerns; families are the most common source of assistance for older adults with cognitive limitations.19,26 Approximately 70%–80% of individuals with a dementing illness live in the community, and, of those individuals, 75% receive care from a family member or friend.19,20,26 Approximately 43.5 million family caregivers care for someone ≥ 50 years of age, and 14.9 million care for someone who has Alzheimer’s disease or another dementing illness.26 Medication management is a complex task that frequently falls within the domain of family caregivers.27 Of the 14.9 million dementia family caregivers in the United States, over half report aiding with medication management at some point in the care process,27–31 with resulting hassles and increased caregiver strain.32 Caregivers who manage medications must be aware of scheduling logistics, safety issues such as identifying negative side effects, knowing what to do in an emergency, issues of polypharmacy, and information seeking when appropriate.30 If 1 or more of these processes is not well managed, the result can have negative physical consequences for the care recipient, as well as financial and emotional consequences for the caregiver.9,10,24,33
It is estimated that < 50% of cases of neurocognitive disorders in the United States are ever formally recognized or diagnosed.34 Diagnosis of a neurocognitive disorder is often delayed approximately 6 years after symptom onset, and a consultation is generally sought only after significant impairment of function is recognized.34 Once an individual is “diagnosed,” less than 35% of those with Alzheimer’s disease or other neurocognitive disorders have the formal diagnosis in their medical record.35,36 Those with undiagnosed neurocognitive disorders see 2.5 to 3.4 times more hospital admissions and 3.2 times higher hospital costs than those who have a diagnosed and managed condition.37 Without a clear diagnostic picture, it may be uncertain when family involvement becomes necessary, and many caregivers may be confused as to what level of care to provide and which activities to manage.
The simplest solution to improving medication management in older adults with cognitive difficulties may be early detection of the disease process.38 Early detection has the potential to increase ease of medication management and subsequent adherence for a number of reasons. Early detection also opens the door for continued dialogue between health professionals and caregivers/care recipients28 and may spur patients and caregivers to seek outside support systems to aid in medication management.
This study explored the differences between informal caregivers who aid someone with a known neurocognitive disorder diagnosis and caregivers who report their loved one to have the same level of cognitive impairment but no diagnosis. We examined the hypothesis that there is an association between a known neurocognitive disorder diagnosis in older adults and heightened caregiver confidence for medication management, as well as increased positive medication management behaviors from the caregiver.
METHOD
Study Design
Confidential computerized survey data were collected from 112 women providing at least 2 forms of medication assistance to a family member aged ≥ 60 years from May 2012 to May 2013. Caregivers completed online self-assessments of medication refill and adherence patterns, complexity of medication regimens for the care recipient, behavioral strategies used for medication management, caregiving self-efficacy, coping, depression, and demographic characteristics. All caregivers were required to respond in the affirmative on an online consent form. Following completion of the online survey, participants entered a separate website and provided their contact information in order to receive a $20 gift card as an incentive for their participation. Survey data were collected separately from contact information so that these data were unable to be directly linked. Institutional review board approval was obtained for this study.
Participants
Inclusion criteria. Prospective participants for inclusion in the survey were (1) women aged ≥ 18 years who were (2) responsible for a family member or friend aged ≥ 60 years who was (3) living in the community. Caregivers were eligible if they were engaging in at least 2 of the following care activities related to prescription drugs: overseeing or planning administration schedules, administering medications, and making or sharing decision making with care recipient and physician to begin, hold, increase, decrease, or discontinue a medication. Similar criteria have been used successfully in research on medication administration by family members of older adults.30 For the purposes of this article, caregivers were included in data analyses if they were caring for someone with a reported analog Clinical Dementia Rating (CDR) score between 0.5 and 1; older adults varied as to whether they had a known neurocognitive disorder diagnosis. Of the 253 caregivers screened, 196 were eligible for the study and provided consent to participate (47%); 112 of the total 253 participants were included in the data analysis due to a reported CDR score indicating mild possible dementia (44%; 0.5–1).
As shown in Table 1, 70% of this sample reported providing care for a parent, 17% for a spouse, and 7% for a grandparent. Caregivers estimated providing approximately 74% of all care needed/provided for the older adult. The mean age of women in this sample was approximately 49 years (SD = 14.20), with a mean education of about 16 years (SD = 2.71). The majority of this sample was white (74.8%) followed by black (20.7%). There were no significant demographic differences in those who cared for an older adult with a neurocognitive disorder diagnosis and those who cared for an older adult without such a diagnosis on percentage of care provided, impairment in instrumental and basic activities of daily living, or the other aforementioned demographic variables.
Measures
Demographics. A brief demographics questionnaire included questions about caregiver and care recipient ethnicity, marital status, education, age, percentage of care provided to the care recipient, and known care recipient neurocognitive disorder diagnosis status (Table 1).
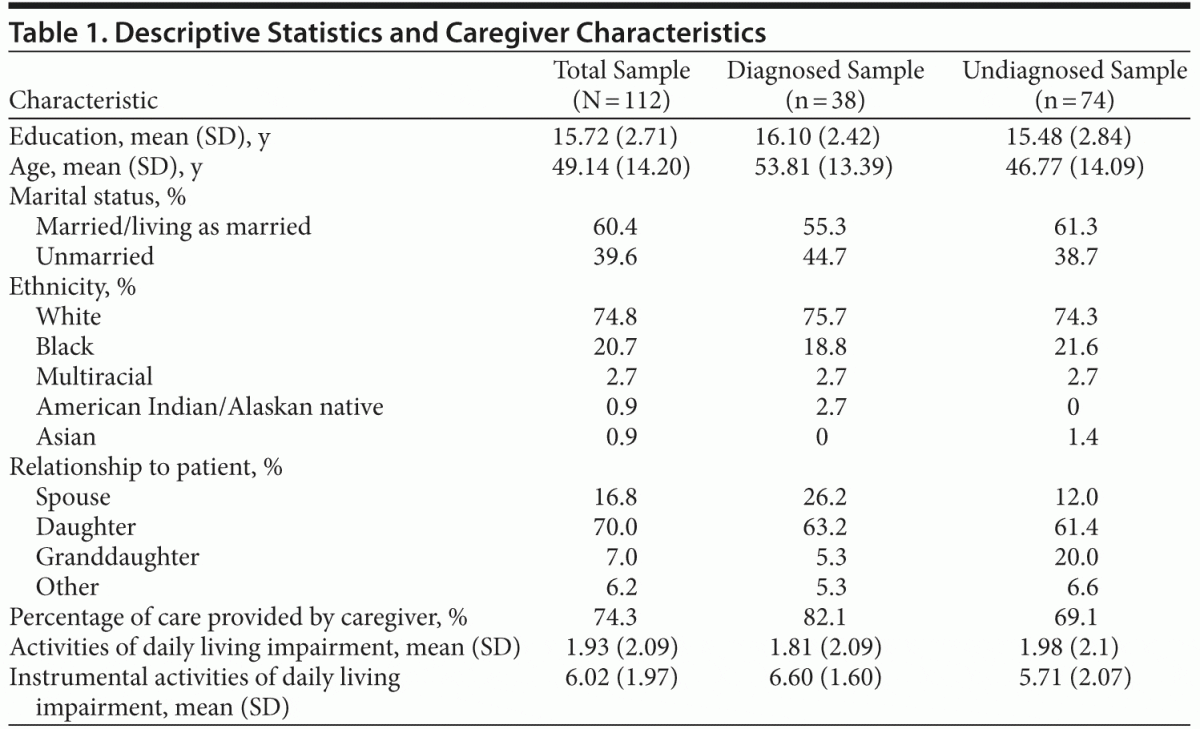
Clinical Dementia Rating. Caregivers reported on the older adult’s impairment related to memory and cognitive problems using an analog version of the CDR. Caregivers were asked about the care recipients’ limitations in these domains only as they related to cognitive functioning (and not due to possible physical limitations). Although the CDR is traditionally administered as a face-to-face structured interview, it was modified in this case for online caregiver self-report. The CDR uses a 5-point rating scale, with total scores ranging from 0 to 3.39 A score of 0 indicates no current impairment, a score of 0.5 indicates very mild possible impairment, and a score of 1, 2, or 3 indicates mild, moderate, or severe cognitive impairment, respectively. The CDR measures 6 domains of cognitive impairment: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal affairs; an online scoring program is available to translate individual ratings into a final impairment score using a detailed algorithm.
Adherence to Refills and Medications Schedules. Caregivers reported their medication administration and refill adherence behaviors relative to the care recipient using the Adherence to Refills and Medications Schedules. This measure consists of 12 items that measure 2 domains of medication behaviors: administering the medication as prescribed and refilling the medication as prescribed.40 A modified 6-item version of this scale was used for the caregiver assessment with 3 items from both the administration and refill adherence domains. Each response was measured on a 4-point Likert scale with the choices “none of the time,” “some of the time,” “most of the time,” or “all of the time.” Item scores were obtained by averaging caregiver responses; higher scores indicate greater levels of nonadherence.
Self-Efficacy for Appropriate Medication Use Scale. This measure asked caregivers about the level of confidence in performing certain adaptive medication management behaviors for their loved one. The study used a modified 5-item version of the 13-item Self-Efficacy for Appropriate Medication Use Scale.41 Questions were tailored such that they asked about a caregiver’s confidence in performing a medication-related activity for/with their older loved one (eg, “How confident are you that you can help the older adult keep taking all of his/her medications as scheduled?”). Caregivers were able to rate their level of confidence from 0% confidence to 100% confidence in increments of 10, and final item scores were obtained by averaging caregiver responses.
RESULTS
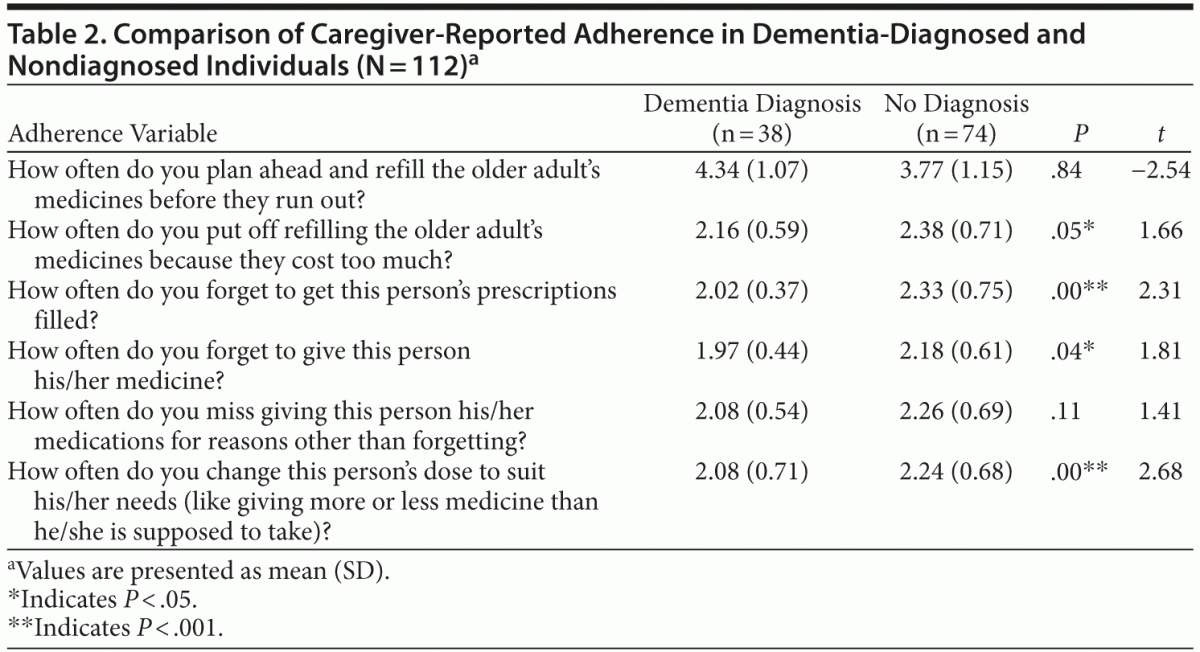
Hypothesis 1, that caregivers with a diagnosed care recipient would report higher medication adherence behaviors than caregivers in nondiagnosed dyads, was tested using an independent samples t test. Participants were grouped by the presence and absence of a reported neurocognitive disorder diagnosis in the care recipient and compared on 6 self-reported medication adherence variables. Approximately 34% of caregivers reported a known dementia diagnosis (n = 38), while 66% denied a known diagnosis but endorsed mild cognitive impairment (n = 74). Analysis revealed a statistically significant difference between the diagnosed and undiagnosed group on several adherence variables (Table 2). Caregivers who were aware of a neurocognitive disorder diagnosis reported fewer occasions of forgetting to refill the older adult’s medicines (mean = 2.02, SD = 0.37) than those caring for a nondiagnosed individual (mean = 2.33, SD = 0.75, t112 = 2.31, P < .001). Caregivers reporting a neurocognitive disorder diagnosis for the patient also endorsed fewer occasions of forgetting to give this person his/her medicine (mean = 1.97, SD = 0.44) than caregivers of nondiagnosed patients (mean = 2.18, SD = 0.61, t112 = 1.81, P < .05). Finally, caregivers of an individual diagnosed with a neurocognitive disorder reported that they were less likely to “change this person’s dose to suit his/her needs (like giving more or less medicine than he/she is supposed to take)” (mean = 2.08, SD = 0.71) than those who did not care for a diagnosed individual (mean = 2.24, SD = 0.68, t112 = 2.68, P < .001).
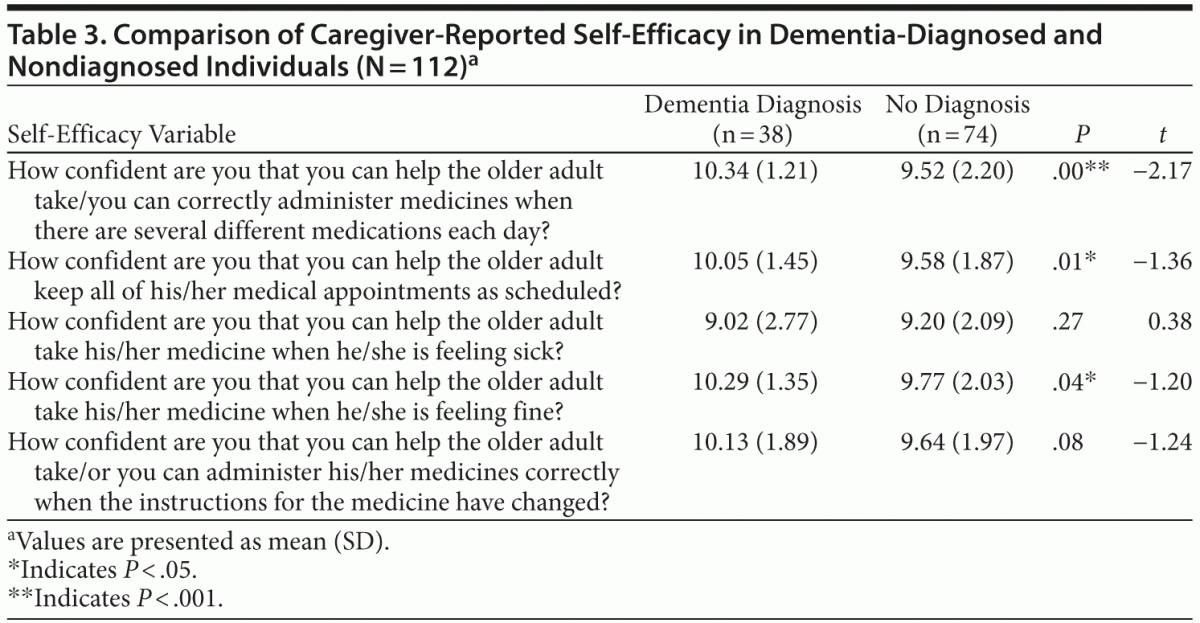
Hypothesis 2, that caregivers with a diagnosed care recipient would report higher self-efficacy for positive medication management behaviors than caregivers in nondiagnosed dyads, was tested with an independent sample t test. Participants were grouped by the reported presence or absence of a neurocognitive disorder diagnosis in the care recipient and compared on 5 self-efficacy variables (Table 3). Analyses revealed that caregivers in the diagnosed group were more likely to feel confident in correctly administering several different medicines each day (diagnosed: mean = 10.34, SD = 1.21; undiagnosed: mean = 9.52, SD = 2.20; t112 = −2.17; P < .001), helping the older adult keep medical appointments as scheduled (diagnosed: mean = 10.05, SD = 1.45; undiagnosed: mean = 9.58, SD = 1.87; t112 = −1.36; P < .05), and administering/helping the older adult take medications when he/she is feeling fine (diagnosed: mean = 10.29, SD = 1.35; undiagnosed: mean = 9.77, SD = 2.03; t112 = −1.20; P < .05).
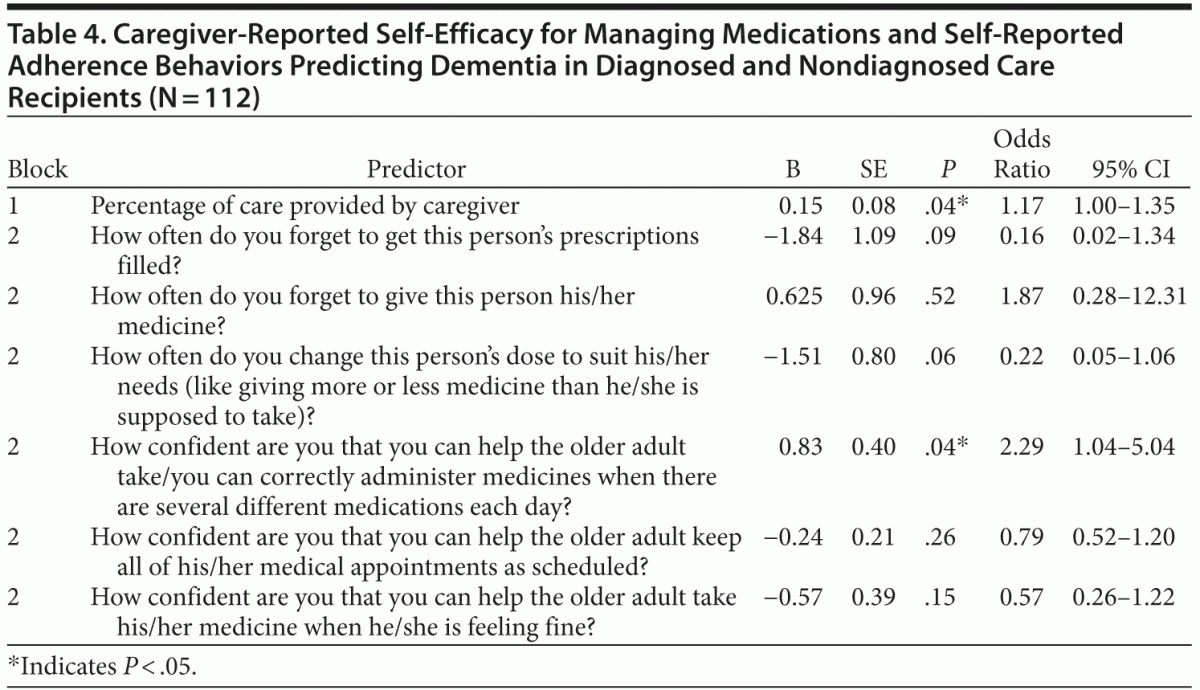
Hypothesis 3 posited that the 6 significant adherence and self-efficacy variables revealed in the 2 previous analyses would predict, at a statistically significant level, the dichotomous neurocognitive disorder diagnosis (ie, yes = 1, no = 0). A stepwise logistic regression was completed using the covariate “percentage of care provided” in the first block and the 6 aforementioned adherence and self-efficacy variables; educational attainment, income, and ability to perform instrumental and basic activities of daily living were removed as covariates due to a lack of statistical significance. Results of the logistic analysis, shown in Table 4, indicate that the 6-predictor step-wise model provides a statistically significant improvement over the covariate alone and constant model (χ26 = 22.84, n = 112, P < .05). The Nagelkerke pseudo R2 indicates that the model accounted for 26.5% of the total variance. The prediction success rate was relatively high for cases used in this model at approximately 70%. Table 4 presents the regression coefficients.
DISCUSSION
To our knowledge, this is the first study to investigate the link between neurocognitive disorder diagnostic status of a care recipient and medication support behaviors by caregivers. Results showed that caregivers with diagnosed loved ones were more likely to engage in positive adherence/administration behaviors and were more confident in their ability to perform certain medication management behaviors. When included in a logistic regression, these adherence behaviors and confidence levels were able to correctly classify, at a significant level, whether or not families were aware of a neurocognitive disorder diagnosis in the older care recipient.
This information highlights the importance of obtaining a clear diagnostic picture when caregivers provide medication aid to an older adult with cognitive impairment. Nonadherence has the potential for devastating physical and financial consequences4,7,9–13 that can impact both the care recipient and the caregivers.28–32 Primary care physicians are often the first to see and assess for cognitive concerns.42 In order to facilitate improved medication adherence, we recommend early detection and diagnosis of neurocognitive disorders through routine early screening and support recommendations that caregivers be a part of this educational process.15
Our findings are in line with recent works suggesting that caregivers who are aware of a diagnosis report increased knowledge of care recipient medical concerns as well as a heightened ability to identify community and interpersonal resources for themselves and the care recipient.43 Limitations of the study include the cross-sectional nature of the data. At present, our study demonstrates an association between neurocognitive disorder diagnosis and heightened caregiver confidence and improved medication management behaviors. Nevertheless, it is feasible that these caregivers have higher overall levels of health care vigilance and that this is the reason that they obtained a diagnosis for the care recipient and why they report more medication management confidence and positive behaviors. It could also be true that prior medication nonadherence led to the older adult developing a neurocognitive disorder or that differing levels of caregiver motivation for improved care initially impacted help-seeking behaviors and continued to impact medication management behaviors. Conversely, it may be the case that having a clear diagnosis underscores the importance of oversight for care recipient health and prompts information seeking for improved medication-related confidence and behaviors. In the future, a prospective study would be helpful in assessing the causal nature of this relationship. Noting relevant changes in caregiver confidence for medication management and changes in management behaviors prediagnosis and postdiagnosis would provide stronger evidence of a causal link.
Author affiliations: Department of Psychology, University of Missouri, St Louis.
Potential conflicts of interest: None reported.
Funding/support: This study was funded by the UM-St Louis Express Scripts Research Program, St Louis, Missouri.
Role of the sponsor: The funding source did not have any role in the design or conduct of the study; collection, management, analysis, or interpretation of data; or in preparation, review, or approval of the manuscript.
REFERENCES
1. Fine RN, Becker Y, De Geest S, et al. Nonadherence consensus conference summary report. Am J Transplant. 2009;9(1):35–41. doi:10.1111/j.1600-6143.2008.02495.x PubMed
2. Center for Disease Control and Prevention and the Merck Company Foundation. The state of aging and health in America. 2007. www.cdc.gov/aging. Updated August 13, 2014. Accessed July 8, 2012.
3. Ferrini A, Ferrini R. Health in the Later Years. 3rd ed. Boston, MA: McGraw Hill; 2000.
4. Banning M. A review of interventions used to improve adherence to medication in older people. Int J Nurs Stud. 2009;46(11):1505–1515. doi:10.1016/j.ijnurstu.2009.03.011 PubMed
5. Barat I, Andreasen F, Damsgaard EM. Drug therapy in the elderly: what doctors believe and patients actually do. Br J Clin Pharmacol. 2001;51(6):615–622. doi:10.1046/j.0306-5251.2001.01401.x PubMed
6. Roth MT, Ivey JL. Self-reported medication use in community-residing older adults: a pilot study. Am J Geriatr Pharmacother. 2005;3(3):196–204. doi:10.1016/S1543-5946(05)80026-1 PubMed
7. DiMatteo MR, Giordani PJ, Lepper HS, et al. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi:10.1097/00005650-200209000-00009 PubMed
8. Fried LP, Walston J. Frailty and failure to thrive. In: Hazzard WR, Blass IP, Halter IB, et al, eds. Principles of Geriatric Medicine and Gerontology. 5th ed. New York, NY: McGraw-Hill; 2003:1487–1502.
9. Bates DW. Preventing medication errors: a summary. Am J Health Syst Pharm. 2007;64(suppl 9):S3–S9, quiz S24–S26. doi:10.2146/ajhp070190 PubMed
10. Berry SD, Quach L, Proctor-Gray E, et al. Poor adherence to medication may be associated with falls. J Gerontol. 2010;65(5):553–558. doi:10.1093/gerona/glq027 PubMed
11. Klarin I, Wimo A, Fastbom J. The association of inappropriate drug use with hospitalization and mortality: a population-based study of the very old. Drugs Aging. 2005;22(1):69–82. doi:10.2165/00002512-200522010-00005 PubMed
12. New England Healthcare Institute. Thinking outside the pillbox: a system-wide approach to improving patient medication adherence for chronic disease. 2009. http://www.nehi.net/publications/17-thinking-outside-the-pillbox-a-system-wide-approach-to-improving-patient-medication-adherence-for-chronic-disease/view. Updated August 12, 2009. Accessed July 19, 2012.
13. Patel RP, Taylor SD. Factors affecting medication adherence in hypertensive patients. Ann Pharmacother. 2002;36(1):40–45. doi:10.1345/aph.1A046 PubMed
14. Douglas A, Letts L, Richardson J. A systematic review of accidental injury from fire, wandering and medication self-administration errors for older adults with and without dementia. Arch Gerontol Geriatr. 2011;52(1):e1–e10. doi:10.1016/j.archger.2010.02.014 PubMed
15. Desai AK, Grossberg GT. Recognition and management of behavioral disturbances in dementia. Prim Care Companion J Clin Psychiatry. 2001;3(3):93–109. doi:10.4088/PCC.v03n0301 PubMed
16. Col N, Fanale JE, Kronholm P. The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly. Arch Intern Med. 1990;150(4):841–845. doi:10.1001/archinte.1990.00390160093019 PubMed
17. Chia LR, Schlenk EA, Dunbar-Jacob J. Effect of personal and cultural beliefs on medication adherence in the elderly. Drugs Aging. 2006;23(3):191–202. doi:10.2165/00002512-200623030-00002 PubMed
18. Brauner D. Adherence to medication in patients with dementia: problems and solutions. Geriatrics & Aging. 2009;12(5):259–263.
19. Schulz R, Martire LM. Family caregiving of persons with dementia: prevalence, health effects, and support strategies. Am J Geriatr Psychiatry. 2004;12(3):240–249. doi:10.1097/00019442-200405000-00002 PubMed
20. US Census Bureau. 65+ in the United States: 2005. Washington, DC; 2005. http://www.census.gov/prod/2006pubs/p23-209.pdf. Accessed September 9, 2014.
21. Insel K, Morrow D, Brewer B, et al. Executive function, working memory, and medication adherence among older adults. J Gerontol B Psychol Sci Soc Sci. 2006;61(2):102–107. doi:10.1093/geronb/61.2.P102 PubMed
22. Stoehr GP, Lu S-Y, Lavery L, et al. Factors associated with adherence to medication regimens in older primary care patients: the Steel Valley Seniors Survey. Am J Geriatr Pharmacother. 2008;6(5):255–263. doi:10.1016/j.amjopharm.2008.11.001 PubMed
23. Field TS, Mazor KM, Briesacher B, et al. Adverse drug events resulting from patient errors in older adults. J Am Geriatr Soc. 2007;55(2):271–276. doi:10.1111/j.1532-5415.2007.01047.x PubMed
24. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157–1165. doi:10.1056/NEJMra052321 PubMed
25. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723–1732. doi:10.1046/j.1532-5415.2002.50468.x PubMed
26. Alzheimer’s Association. Changing the Trajectory of Alzheimer’s Disease: A National Imperative. Washington, DC: Public Policy Office; 2010.
27. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Alzheimer’s Disease and Other Dementias. Arlington, VA: American Psychiatric Association; 2010.
28. Alzheimer’s Association. Types of dementia. 2012. http://www.alz.org/dementia/types-of-dementia.asp. Accessed July 8, 2013.
29. National Alliance for Caregiving in Collaboration with AARP. Caregiving in the US 2009. http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf. Accessed January 25, 2013.
30. Travis SS, Kao HF, Acton GJ. Helping family members manage medication administration hassles. J Psychosoc Nurs Ment Health Serv. 2005;43(11):13–15. PubMed
31. Arlt S, Linder R, Rosler A, et al. Adherence to medication in persons with dementia: predictors and strategies for improvement. Drugs Aging. 2008;25(12):1033–1047. doi:10.2165/0002512-200825120-00005 PubMed
32. Thornton M, Travis SS. Analysis of the reliability of the modified Caregiver Strain Index. J Gerontol B Psychol Sci Soc Sci. 2003;58(2):S127–S132. doi:10.1093/geronb/58.2.S127 PubMed
33. Murray MD, Callahan CM. Improving medication use for older adults: an integrated research agenda. Ann Intern Med. 2003;139(5 pt 2):425–429. doi:10.7326/0003-4819-139-5_Part_2-200309021-00009 PubMed
34. Silverstein NM, Maslow K, eds. Improving Hospital Care in People With Dementia. New York, NY: Springer; 2006:3–21.
35. Boustani M, Callahan CM, Unverzagt FW, et al. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20(7):572–577. doi:10.1007/s11606-005-0103-7 PubMed
36. Boise L, Neal MB, Kaye J. Dementia assessment in primary care: results from a study in three managed care systems. J Gerontol A Biol Sci Med Sci. 2004;59(6):M621–M626. doi:10.1093/gerona/59.6.M621 PubMed
37. US Center for Medicare and Medicaid. Medicaid Offices. 2000. http://www.cms.gov/Medicare/Coverage/ClinicalTrialPolicies/index.html?redirect=/clinicaltrialpolicies/. Updated June 28, 2013. Accessed March 13, 2013.
38. Topinková E, Baeyens JP, Michel JP, et al. Evidence-based strategies for the optimization of pharmacotherapy in older people. Drugs Aging. 2012;29(6):477–494. doi:10.2165/11632400-000000000-00000 PubMed
39. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi:10.1212/WNL.43.11.2412-a PubMed
40. Kripalani S, Risser J, Gatti ME, et al. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health. 2009;12(1):118–123. doi:10.1111/j.1524-4733.2008.00400.x PubMed
41. Risser J, Jacobson TA, Kripalani S. Development and psychometric evaluation of the Self-Efficacy for Appropriate Medication Use Scale (SEAMS) in low-literacy patients with chronic disease. J Nurs Meas. 2007;15(3):203–219. doi:10.1891/106137407783095757 PubMed
42. Forester BP, Oxman TE. Measures to assess the noncognitive symptoms of dementia in the primary care setting. Prim Care Companion J Clin Psychiatry. 2003;5(4):158–163. doi:10.4088/PCC.v05n0403 PubMed
43. Galvin JE, Tolea MI, George N, et al. Public-private partnerships improve health outcomes in individuals with early stage Alzheimer’s disease. Clin Interv Aging. 2014;9:621–630. doi:10.2147/CIA.S60838 PubMed
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top

