Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
Ms A is a 50-year-old white woman who presented to the Stead Family Memory Center at Banner Alzheimer’s Institute for evaluation of cognitive impairment in 2012. The patient reported a 3-year history of insidious onset of short-term memory deficits and difficulties with concentration. Ms A reported that she had struggled with multiple psychiatric conditions including bipolar II disorder (diagnosed in 1999) with related anxiety and prominent depressive features.

CASE CONFERENCE
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discuss challenging and/or teaching cases of patients seen at the Institute’s Memory Disorders Clinic. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), physician assistants, social workers, nurses, medical students, residents, and fellows.
BANNER ALZHEIMER’ S INSTITUTE
The Banner Alzheimer’s Institute located in Phoenix, Arizona, has an unusually ambitious mission: to end Alzheimer’s disease without losing a generation, set a new standard of care for patients and families, and forge a model of collaboration in biomedical research. The Institute provides high-level care and treatment for patients affected by Alzheimer’s disease, dementia, and related disorders. In addition, the Institute offers extensive support services for families and many unique and rewarding research opportunities.
Prim Care Companion CNS Disord 2017;19(2):17alz02131
https://doi.org/10.4088/PCC.17alz02131
© Copyright 2017 Physicians Postgraduate Press, Inc.
Submitted: March 10, 2017; accepted March 14, 2017.
Published online: April 27, 2017.
Anna D. Burke, MD, is a geriatric psychiatrist and dementia specialist at the Stead Family Memory Clinic of Banner Alzheimer’s Institute and a clinical assistant professor of psychiatry at the University of Arizona College of Medicine, Phoenix.
William J. Burke, MD, is a geriatric psychiatrist and the director of the Stead Family Memory Clinic of Banner Alzheimer’s Institute and a research professor of psychiatry at the University of Arizona College of Medicine, Phoenix.
Garrett H. Riggs, MD, is a behavioral neurologist and dementia specialist at the Stead Family Memory Clinic of Banner Alzheimer’s Institute.
David A. Weidman, MD, is a neurologist and dementia specialist at the Stead Family Memory Clinic of Banner Alzheimer’s Institute.
Jacquelynn N. Copeland, PhD, is a neuropsychologist at Banner Alzheimer’s Institute.
Corresponding author: Anna D. Burke, MD, Banner Alzheimer’s Institute, 901 E Willetta St, Phoenix, AZ 85006 ([email protected]).
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. This special series of case reports about dementia was deemed valuable for educational purposes by the Publisher, Editor in Chief, and CME Institute Staff. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation.
CME Objective
After studying this article, you should be able to:
- Assess younger adult patients with cognitive problems and mood disorders for dementia
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Date of Original Release/Review
This educational activity is eligible for AMA PRA Category 1 Creditâ„¢ through April 30, 2019. The latest review of this material was April 2017.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for Allergan, Ironshore, Lundbeck, Shire, and Sunovion; has been a stock shareholder of M-3 Information; and has received royalties from UpToDate and Oxford University Press. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears on the next page.
HISTORY OF PRESENTING ILLNESS
Ms A is a 50-year-old white woman who presented to the Stead Family Memory Center at Banner Alzheimer’s Institute for evaluation of cognitive impairment in 2012. The patient reported a 3-year history of insidious onset of short-term memory deficits and difficulties with concentration. Ms A reported that she had struggled with multiple psychiatric conditions including bipolar II disorder (diagnosed in 1999) with related anxiety and prominent depressive features. Her symptoms were difficult to control, and she failed several psychopharmacologic trials in the past. In addition, she reported some evidence of cluster B personality traits including tendency toward impulsivity, emotional lability, and self-injurious behaviors. As a result of her psychiatric conditions, Ms A was on a complex psychopharmacologic regimen and was undergoing psychotherapy with an outside therapist. In addition, she was dealing with the stress of being a caregiver for her mother who was diagnosed with Alzheimer’s dementia approximately 3 years ago at Banner Alzheimer’s Institute.
Bipolar I disorder is characterized by 1 or more manic episodes or mixed episodes (when the patient experiences symptoms of both mania and depression). Patients will also typically experience periods of depression. Bipolar I disorder is marked by extreme manic episodes that interfere with functioning. Bipolar II disorder is diagnosed after 1 major depressive episode or more and at least 1 episode of hypomania. The “highs” in bipolar II disorder are not as severe as in bipolar I disorder and may not significantly impact a patient’s functioning.
Personality disorders are enduring patterns of inner experience and behavior that deviate markedly from the expectations of an individual’s culture and have onset in adolescence or early adulthood. They are stable over time and can lead to distress or impairment. Personality disorders and the traits related to them can be divided into 10 specific disorders; these disorders can further be divided into clusters. Cluster A includes paranoid, schizoid, and schizotypal personality disorders. Cluster B includes antisocial, borderline, histrionic, and narcissistic personality disorders. Cluster C includes avoidance, dependent, and obsessive-compulsive personality disorders.
Ms A reported that when her symptoms began, she started to forget details of conversations and events. She denied misplacing items around the house but did report having more difficulty tracking appointments and dates. She was more repetitive in statements and questions. She endorsed occasional difficulty with word finding. She experienced difficulty focusing on what she was reading, and, occasionally, her writing appeared shakier. She had developed some mild extrapyramidal symptoms according to her partner of 20 years, including a tremor, flattening of facial expression, and a more rigid unstable walk with occasional falls. This change in mobility appeared to coincide with initiation of risperidone. According to her partner, her personality also began to change at this time. She was not as lighthearted as she had been in the past. She appeared more withdrawn, depressed, and fatigued. She was sleeping excessively or hiding in her bedroom even when not asleep. She began to withdraw from activities she had enjoyed in the past such as making gourd art and painting. She began to have difficulty with focus and could not readily remember people’s names or phone numbers. She also was noted to have become increasingly irritable over that period of time.
Ms A reported that she had more difficulty controlling her depressive episodes. She had 3 episodes of increased suicidal ideations with 2 suicide attempts, both via overdose, over the past 2 to 3 years. She reported that both times her intent was to take her own life, but not so that others could understand her pain. She reported hypomanic episodes during which she read multiple books at once. Her mood during these episodes was upbeat and jovial. She reported being more impulsive, taking on numerous projects, and displaying more risk-taking behavior. Her thoughts appeared to race, and her speech was pressured during these times. These episodes were followed by frequent depressive episodes meeting full DSM-5 criteria for major depressive disorder. She was working with her psychiatrist to stabilize her affective symptoms. Her psychiatrist felt that her fluctuations in cognition were partly related to adjustments to her medication regimen. Ms A was started on donepezil by her psychiatrist who felt that it might improve her focus. She reported some improvement in cognitive clarity since starting the medication but continued to have difficulty with focus.
It is important to distinguish between suicide attempts versus suicidal gestures. Individuals who suffer from certain cluster B personality disorders have a greater tendency toward suicidal gestures. Suicidal gestures are attempts to convert the emotional pain they feel into something real. These individuals also may want others to understand how much emotional distress they are in or may want to avoid abandonment. Suicidal gestures may include, for example, overdoses on medications that are strategically scheduled for a time when a loved one will return to find the victim. Suicidal gestures also may take the form of self-injurious behaviors such as cutting or other physical injury. It is vital to question patients about the intent of their suicide attempt.
Ms A’s family reported occasional irritability over the past year and a half that had improved slightly with medication adjustments. The patient reported a history of cutting and hitting herself and physically aggressive behaviors toward her partner, but there was no agitation or verbal aggression at the time of evaluation, and physically aggressive behaviors had resolved. No psychotic symptoms were reported.
From a functional standpoint, Ms A did not manage her finances. She required assistance with medications due to forgetting whether she had taken the medications and previous suicide attempt via overdose. She continued to be able to calculate tips and make correct change. She made lists and followed them when shopping but had a tendency to impulsively buy items that she did not need or to buy too much of something. The family reported that she continued to drive with no difficulty. She had limited motivation in doing household chores but could still cook meals. Occasional confusion regarding the TV remote was present but minimal. No changes in personal hygiene or grooming were noted. No urinary or fecal incontinence was present.
PAST MEDICAL HISTORY
Ms A reported a history of bipolar II disorder, lap band surgery, hysterectomy, and tonsillectomy.
ALLERGIES
There were no known drug allergies.
MEDICATIONS
Current medications included lithium 300 mg twice daily, risperidone 2 mg daily, donepezil 10 mg daily, citalopram 20 mg daily, benztropine 0.5 mg twice daily, and alprazolam 2 mg at night. Ms A reported taking an additional 1-mg dose of alprazolam during the day on rare occasion if anxiety was overwhelming.
SOCIAL HISTORY
Ms A is a high school graduate and worked as a travel consultant for approximately 18 years. She was on disability for the past 10 years due to her psychiatric illness. She lives independently in her own home with her partner. The patient’s mother, who has moderately severe Alzheimer’s disease, lives with them.
SUBSTANCE ABUSE HISTORY
There was no known history of alcohol or illicit drug abuse. The patient smoked approximately one-and-a-half packs of cigarettes per day prior to quitting in August 2002. She smoked for 25 years.
FAMILY HISTORY
The patient’s mother began to experience short-term memory loss at the age of 73 years before receiving a diagnosis of Alzheimer’s dementia. Her mother, father, and brother suffered from mental illness, mainly clinical depression. A positive history of suicide was also present in the family. There was no known family history of Parkinson’s disease or stroke.
MENTAL STATUS EXAMINATION
The patient was well groomed and appeared to be in no acute distress. She displayed decreased blink and facial expressions. Some psychomotor slowing was noted; her eye contact was otherwise appropriate. She was pleasant and cooperative with the examination. Affect was noted to be constricted. Mood was good. Thought process was coherent, logical, and goal directed. There was no evidence of any paranoid, suicidal, or homicidal ideations. No auditory or visual hallucinations were noted. Speech was of normal volume, rate, and amount. Judgment and insight were good. Orientation to time, place, and person was maintained as was recent and remote memory. Attention and concentration was slightly impaired. Fund of knowledge was appropriate for age and educational level.
On the basis of the information so far, what would you expect to see on the neurologic examination?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Normal | 25% |
| B. Objective nonfocal neurologic findings (including frontal release signs) | 75% |
| C. Focal neurologic findings | 0% |
Most of the participants felt that the neurologic examination would be essentially normal, although some mild extrapyramidal symptoms might be present as a result of the risperidone use.
PHYSICAL AND NEUROLOGIC EXAMINATION
Ms A’s physical examination showed mild abnormalities but no localizing or lateralizing findings. The patient was obese. A mild intention tremor was noted bilaterally in the upper extremities, right more than left. She displayed a wide-based gait and had some difficulty with tandem gait.
On the basis of the information so far, do you think a major neurocognitive disorder is present?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Yes | 25% |
| B. No | 50% |
| C. Not enough information | 25% |
Most participants felt that there was not enough functional change related to cognitive disturbance to warrant a diagnosis of a major neurocognitive disorder. Some participants commented that additional information was necessary, as the patient’s medications may be affecting her cognition and function.
The DSM-5 (American Psychiatric Association, 2013) defines a major neurocognitive disorder as follows:
- Evidence of significant cognitive decline from a previous level of performance in 1 or more areas of cognitive domains (complex attention, executive function, learning and memory, language, perceptual-motor or social cognition) based on:
- Concern of the individual, a knowledgeable informant, or the clinician that there has been a significant decline in cognitive function and
- Substantial impairment in cognitive performance, preferably documented by standardized neuropsychological testing or, in its absence, another quantified clinical assessment.
- The cognitive deficits interfere with independence in everyday activities.
- The cognitive deficits do not occur exclusively in the context of a delirium.
- The cognitive deficits are not better explained by another mental disorder.
LABORATORY AND RADIOLOGY RESULTS
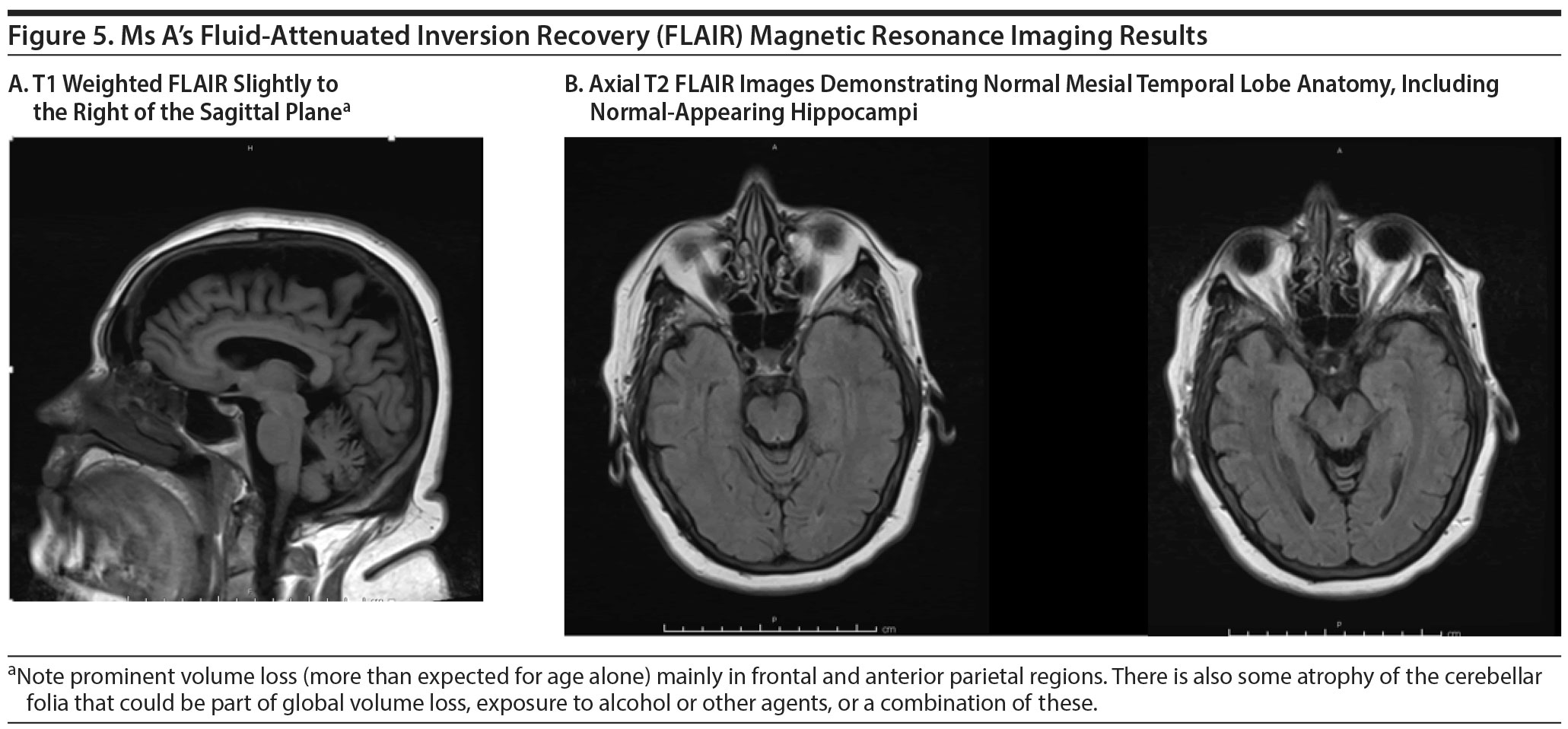
A magnetic resonance image (MRI) available at the time of the visit revealed small-vessel ischemic changes and cerebral atrophy more notable in the bilateral frontal lobes. There was no evidence of acute intracranial pathology. Laboratory studies including complete blood count with differential, a comprehensive metabolic panel, vitamin B12 level, thyroid-stimulating hormone level, lithium level, and urinalysis and urine culture showed no clinically significant abnormalities.
On the basis of the information so far, what would you expect the MMSE score to be?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. | 26-30 | 25% |
| B. | 21-25 | 75% |
| C. | 16-20 | 0% |
| D. | 11-15 | 0% |
| E. | < 11 | 0% |
Ms A scored 27/30 on the MMSE (Folstein et al, 1975), losing 2 points on attention and calculation and 1 point on design copy. The expected minimum score for Ms A’s age and education is 26.
On the basis of the information so far, what would you expect the MoCA score to be?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. 26-30 | 0% |
| B. 21-25 | 100% |
| C. 16-20 | 0% |
| D. 11-15 | 0% |
| E. < 11 | 0% |
Ms A scored 20/30 on the MoCA (Nasreddine et al, 2005) (expected norms not defined for this demographic) (Figure 1). Impairments were noted mainly in attention and concentration, phonemic fluency, and abstraction. It is notable that Ms A was able to recall 2 of the 5 words she was asked to remember with no cues but was able to remember the remaining 3 words with cues, indicating that this performance was less likely a reflection of a memory storage deficit and more likely difficulty with retrieval. An auditory verbal learning test revealed that Ms A was able to learn up to 11 of the 15 words over the course of 5 trials with 39 total words learned. Category retrieval was 20 animals over the course of 60 seconds with no repetitions and no errors (expected minimum for age: 19). Delayed recall of visual figures that she had previously copied was 3/3. A geriatric depression scale revealed a score of 13, indicating the presence of continued moderate depressive symptoms. Ms A’s clock drawing is shown in Figure 2.
The Montreal Cognitive Assessment (Nasreddine et al, 2005) has been shown to have a better sensitivity and specificity in detecting more subtle cognitive impairments, such as mild cognitive impairment (MCI), when compared to the Mini-Mental State Examination. Nasreddine et al (2005) found that the MMSE had a sensitivity of 18% to detect MCI, whereas the MoCA detected 90% of MCI subjects. In the mild Alzheimer’s disease group, the MMSE had a sensitivity of 78%, whereas the MoCA detected 100%. Specificity was excellent for both the MMSE and MoCA (100% and 87%, respectively).
On the basis of the information so far, do you think a major neurocognitive disorder is present?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Yes | 25% |
| B. No | 75% |
| C. Not enough information | 0% |
On the basis of the neuropsychological evaluation, participants felt the most likely cause of Ms A’s symptoms was bipolar disorder. Those with depression benefit from cues because multiple choice is inherently an easier and less effortful task. Additionally, qualitatively, Ms A said, “I can’ t do it,” and gave up without trying on the serial 7s, which also may reflect diminished motivation and effort. Some participants felt that the metrics testing suggested a possible mild neurocognitive disorder (mild cognitive impairment range) but not major neurocognitive disorder.
Of potential differential diagnoses, what is the most likely primary etiology of the patient’s current cognitive and behavioral symptoms?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Alzheimer’s disease | 0% |
| B. Frontotemporal lobar degeneration | 25% |
| C. Lewy body disease | 0% |
| D. Vascular disease | 0% |
| E. Bipolar disorder | 25% |
| F. Personality disorder | 0% |
| G. Adjustment disorder with mixed disturbances of emotions and conduct | 0% |
| H. Adverse effects of polypharmacy | 50% |
| I. Malingering | 0% |
Most participants felt that the primary etiology of Ms A’s cognitive impairment was polypharmacy. They believed that Ms A was experiencing the cognitive impact of anticholinergic medications and anxiolytics. They also could not fully rule out the impact of her poorly controlled depressive symptoms.
Some participants felt that further investigation was necessary, as the symptoms of bipolar disorder and a behavioral variant of frontotemporal dementia may overlap. They were concerned about the cerebral atrophy noted on the MRI in the bilateral frontal lobes.
Which of the following evaluations or treatments would you schedule next?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Neuropsychological evaluation | 0% |
| B. FDG-PET scan | 0% |
| C. Amyloid PET scan | 0% |
| D. Work with the patient’s psychiatrist to adjust her medication | 100% |
| E. No further workup is necessary | 0% |
All participants believed that a reduction in anticholinergic and anxiolytic medications was the first step.
TREATING PHYSICIAN’ S IMPRESSION
Taking into account the patient’s history and clinical presentation, the treating physician in 2012 did not believe that Ms A suffered from a neurodegenerative disorder at that time. It was felt that she did not display a typical pattern of cognitive deficits on screening tests or history that would be consistent with an Alzheimer’s -type process. Her cognitive testing and clinical assessment revealed significant impairments in attention that were felt to be causing her memory problems. Frontal lobe atrophy was noted on the MRI and behavioral changes were present; therefore, frontotemporal dementia could be a possibility. However, there was no objective evidence on cognitive screening testing of any significant executive dysfunction or impairments in frontal lobe or temporal lobe function. The physician suspected that Ms A’s current symptoms were largely related to her bipolar disorder and the intense medication regimen necessary to control it, as well as the additional burden of becoming a caregiver for her mother.
The physician recommended a follow-up with Ms A’s psychiatrist to “clean up” the medication regimen. A telephone discussion was held between the physicians, and the following recommendations were made: It was recommended that the necessity for risperidone and benztropine be reviewed, as both of these medications could possibly be negatively affecting the patient’s cognition. Alprazolam was also to be reassessed, as it can result in cognitive clouding. Lithium is also known to cause states of confusion and difficulty with focus. However, it appeared that lithium could not be reduced or eliminated, as it had been the most effective mood-stabilizing agent for Ms A. Decreasing these medications or titrating the patient off them was the goal. If Ms A continued to experience a cognitive decline despite adjustments to her psychopharmacologic regimen, she was asked to contact Banner Alzheimer’s Institute for further evaluation.
Ms A returned to Banner Alzheimer’s Institute in 2016 reporting that over the course of the past several years her memory and focus seemed to gradually worsen. Despite previous recommendations to reduce medications affecting cognition, the patient’s medication regimen became even more robust. During the clinical evaluation, she appeared to be able to give details of recent events including conversations that she had recently had with family members and physicians. However, she did appear to struggle somewhat to express herself. Difficulties with word-finding and maintaining and completing her thoughts were noted.
FOLLOW-UP
Ms A reported that since her last visit she was experiencing an increased number of falls and unsteadiness, prompting her psychiatrist to reduce her alprazolam dose from 1 mg 5 times a day to 1 mg twice a day. This medication change reduced her falls and improved her focus somewhat, but her symptoms did not fully resolve. Ms A found it more difficult to complete sentences and thoughts. She had more difficulty with executive function, leading to more challenges with managing her medication regimen and multitasking. She was experiencing more difficulty using the computer but continued to use household appliances such as the TV remote and telephone appropriately. Her writing had begun to change and was “more sloppy.” No micrographia was noted. She became more lax in personal hygiene and grooming mainly because she did not feel like doing it. Her appetite remained good; however, she reported occasional difficulty with swallowing. A neurologic examination revealed no evidence of fasciculations, significant gait disturbances, or other significant neurologic abnormalities.
Fasciculations or twitching of muscles along with other physical symptoms such as problems with articulation, difficulty with swallowing, gait changes, and wasting or weakness of muscles may indicate the presence of motor neuron disease. Motor neuron disease occurs in 1 of 10 people with frontotemporal dementia, which is a relatively rare form of dementia that can affect behavior, language skills, and cognition. The presence of motor neuron disease has been associated with a more rapid progression of symptoms.
Ms A remained significantly depressed and was now withdrawing from all social settings and gatherings. She avoided public situations. Her partner reported that she was “stressed out” and extremely irritable. Passive suicidal ideations were present, but no active suicidal ideations or plan could be elicited. During the evaluation, Ms A stated that if she were to be diagnosed with a neurodegenerative disorder like her mother, she would become actively suicidal.
Ms A also reported an exacerbation of manic symptoms. She stated that she was “manic every other day.” These episodes were triggered by caregiver stress and interactions with her mother who was now entering the advanced stages of Alzheimer’s dementia. During the manic episodes, Ms A experienced racing thoughts (which she did not feel were related to anxiety), pressured speech, and periods of insomnia. Further investigation of her sleep disturbances indicated that she had a shift in sleep-wake cycle, as she was spending much of her day in bed and was up and about at night.
She admitted to the presence of tactile hallucinations including sensations that someone was rubbing up against her thigh. She occasionally saw shadows or visual hallucinations of dead pets. Her partner described her as becoming more paranoid, particularly of others’ intents.
MEDICATIONS
Medication adjustments had been made since her initial visit to Banner Alzheimer’s Institute. Ms A was taking the following medications: alprazolam 1 mg twice daily, citalopram 40 mg daily, gabapentin 300 mg twice daily, lamotrigine 200 mg nightly, lithium 300 mg twice daily, tramadol 50 mg as needed every 6 hours, quetiapine 25 mg nightly, ibuprofen 600 mg as needed every 6 hours, and omeprazole 10 mg daily. Recent laboratory studies were available and revealed no evidence of any significant abnormalities. In-office cognitive screening tests were performed again.
On the basis of the information so far, what would you expect the MMSE score to be?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. 26-30 | 0% |
| B. 21-25 | 100% |
| C. 16-20 | 0% |
| D. 11-15 | 0% |
| E. < 11 | 0% |
Ms A scored 22/30 on the MMSE. She displayed impairments primarily in attention.
On the basis of the information so far, what would you expect the MoCA score to be?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. 26-30 | 0% |
| B. 21-25 | 100% |
| C. 16-20 | 0% |
| D. 11-15 | 0% |
| E. < 11 | 0% |
Ms A scored 17/30 on the MoCA (Figure 3). During the course of the test, she at times refused to perform some of the tasks, stating “I cannot, I cannot.” Although she was able to learn words (normal registration), free recall was diminished but improved to 5/5 with category or multiple-choice cues (ie, a retrieval-based deficit or reflecting improved performance on less effortful tasks when given cues or multiple choice). Attention, again, appeared to be the greatest area of weakness. Ms A’s clock drawing results are included in Figure 4.
On the basis of the information so far, do you think a major neurocognitive disorder is present?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Yes | 25% |
| B. No | 75% |
| C. Not enough information | 0% |
Most participants did not feel the patient met criteria for a major cognitive impairment. They still questioned the influence of medications and affective illness.
Of potential differential diagnoses, what is the most likely primary etiology of the patient’s current cognitive behavioral symptoms?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Alzheimer’s disease | 0% |
| B. Frontotemporal lobar degeneration | 0% |
| C. Lewy body disease | 0% |
| D. Vascular disease | 0% |
| E. Bipolar disorder | 50% |
| F. Personality disorder | 0% |
| G. Adjustment disorder with mixed disturbances of emotions and conduct | 0% |
| H. Adverse effects of polypharmacy | 50% |
| I. Malingering | 0% |
The participants felt that both polypharmacy and affective illness were the primary causes of Ms A’s cognitive symptoms. At this time, they saw no evidence of a neurodegenerative process.
Which of the following evaluations or treatments would you schedule next?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Neuropsychological evaluation | 75% |
| B. FDG-PET scan | 0% |
| C. Amyloid PET scan | 0% |
| D. CSF analysis | 0% |
| E. Repeat MRI | 0% |
| F. Work with the patient’s psychiatrist to adjust her medication | 25% |
| G. No further workup is necessary | 0% |
Most participants believed that a comprehensive neuropsychological evaluation could establish a pattern of cognitive strengths and weaknesses that could help determine if a neurodegenerative process was present or Ms A’s symptoms were solely related to polypharmacy or bipolar disorder. As an initial step, the participants felt that the testing would provide more useful information than neuroimaging or biomarker testing.
TREATING PHYSICIAN’ S PLAN
The treating physician ordered a neuropsychological evaluation to assess for the presence of a pattern of cognitive strengths and weaknesses that could help clarify the exact etiology of symptoms. This testing would also establish a baseline for future monitoring of progression of illness and help with nonpharmacologic treatment planning by utilizing the patient’s strengths and weaknesses. Cognitive effects of affective illness and polypharmacy were felt to be high on the differential diagnosis, but a neurodegenerative disorder such as frontotemporal dementia was also felt to be a possibility. An FDG-PET scan was also ordered to assess for a pattern of hypometabolism consistent with a frontotemporal-dementing process.
NEUROPSYCHOLOGICAL EVALUATION RESULTS
Neuropsychological evaluation revealed that the patient’s level of intellectual functioning was in the low average range, which appeared roughly consistent with her educational and occupational attainment. She displayed impairments in attention and concentration, which were found to be borderline abnormal. Her speed of information processing was on the low end of average to mildly abnormal. She scored average on confrontation naming tasks but displayed impairments on other assessments of language including verbal fluency, which was moderately abnormal for letters of the alphabet and borderline abnormal for category. She scored average on verbal concept formation, verbal reasoning, and knowledge acquirement from her environment. Her visual perceptual abilities were borderline abnormal. Executive functioning abilities hovered in the low average range. She became easily confused and was unable to complete a task that required her to keep track of 2 sequences simultaneously and shift back and forth between them.
Ms A’s auditory verbal memory abilities were also an area of weakness. When asked to learn and recall a list of 16 words, her total score on learning and immediate recall was moderately abnormal. After a 20-minute delay, her free and cued recall was mildly abnormal. In addition, she made a large number of intrusion errors, “remembering” incorrect words. Her immediate recall of a passage was average. However, after a delay, her recall of this material was borderline abnormal.
The findings of the evaluation were noteworthy for low scores in multiple domains. Because of poor performance on validity measures, the neuropsychologist questioned whether the patient was exerting maximal effort and was fully engaged in the testing to perform at her optimal cognitive level. He also questioned whether the patient’s current cognitive deficits were related to difficulty with focus and attention and whether her medication regimen or affective instability was contributing to the current findings. However, an underlying neurodegenerative process could not be ruled out.
On the basis of the neuropsychological testing results, what is the most likely etiology of the patient’s current cognitive and behavioral symptoms?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Alzheimer’s disease | 0% |
| B. Frontotemporal lobar degeneration | 10% |
| C. Lewy body disease | 0% |
| D. Vascular disease | 0% |
| E. Bipolar disorder | 80% |
| F. Personality disorder | 0% |
| G. Adjustment disorder with mixed disturbances of emotions and conduct | 0% |
| H. Adverse effects of polypharmacy | 10% |
| I. Malingering | 0% |
Most participants believed that the poor effort displayed on neuropsychological evaluation was consistent with bipolar disorder. They felt that bipolar disorder was the primary cause of the patient’s cognitive symptoms. Some participants continued to question the influence of medications and possible frontotemporal lobar degeneration, as both of these conditions can adversely influence motivation.
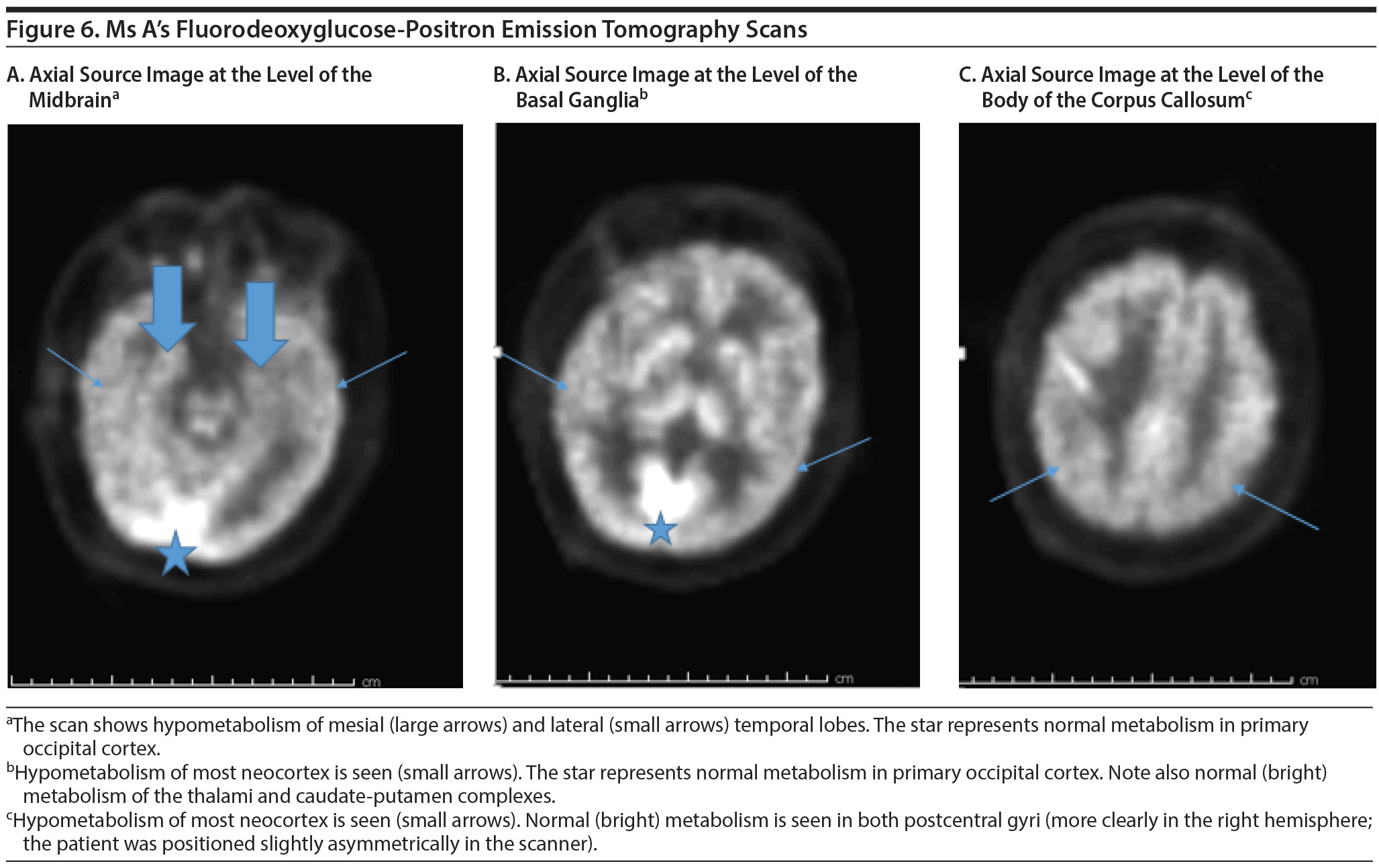
FDG-PET SCAN RESULTS
The FDG-PET scan revealed generalized reduction in cerebral cortical activity bilaterally that appeared symmetric. The reduction was noted inactivity of the temporal lobes, particularly in the hippocampi. The findings were believed to be compatible with a generalized type of Alzheimer’s dementia. Figures 5 and 6 show Ms A’s FDG-PET results.
On the basis of the available workup, what is the most likely primary etiology of the patient’s current cognitive and behavioral symptoms?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Alzheimer’s disease | 100% |
| B. Frontotemporal lobar degeneration | 0% |
| C. Lewy body disease | 0% |
| D. Vascular disease | 0% |
| E. Bipolar disorder | 0% |
| F. Personality disorder | 0% |
| G. Adjustment disorder with mixed disturbances of emotions and conduct | 0% |
| H. Adverse effects of polypharmacy | 0% |
| I. Malingering | 0% |
Most participants were surprised by the neuroimaging results but felt that the pattern of hypometabolism on the FDG-PET was consistent with Alzheimer’s disease. This diagnosis was further reinforced by Ms A’s family history of Alzheimer’s dementia.
What would your next steps in managing this patient be?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Start a cholinesterase inhibitor | 80% |
| B. Start an N-methyl-d-aspartate (NMDA) antagonist | 0% |
| C. Schedule an amyloid PET scan | 0% |
| D. Change citalopram to bupropion | 0% |
| E. Titrate off of alprazolam | 20% |
Most participants felt that starting a cholinesterase inhibitor would be the most appropriate next step in treatment of the Alzheimer’s dementia. Some participants believed that titrating the patient off alprazolam would reduce some of the cognitive clouding and be an appropriate first step prior to initiating a cognitive enhancer.
TREATING PHYSICIAN’ S PLAN
Ms A was diagnosed with Alzheimer’s dementia of mild severity with behavioral disturbances. Although her bipolar disorder and complex medication regimen were most likely contributing to further confusion and cognitive clouding, evidence of an underlying neurodegenerative disorder was present on both FDG-PET scan and structural neuroimaging. The pattern appeared most consistent with Alzheimer’s disease. Ms A was referred for an amyloid PET scan, but the cost of the scan was prohibitive for the patient. It was felt that an amyloid PET scan could further bolster a diagnosis of Alzheimer’s disease, considering the patient’s young age. Ms A was started on donepezil, which had been stopped by her psychiatrist since her initial visit to Banner Alzheimer’s Institute in 2012. Shortly after increasing the dose to 10 mg daily, she developed gastrointestinal distress and was unable to tolerate the medication. She was then switched to the rivastigmine patch but developed severe headaches and had to stop the medication. She was then started on memantine, which was titrated to a final dose of 10 mg twice daily. The patient tolerated this medication well.
Memantine is an N-methyl-d-aspartate (NMDA) receptor antagonist that is currently approved for treatment of Alzheimer’s dementia of moderate to advanced severity. The role of glutamatergic signaling, especially the dysfunction of NMDA receptor complex signaling, appears to overlap in the pathology of Alzheimer’s disease and major depressive disorder. NMDA receptor antagonists have been demonstrated to feature antidementia and antidepressant effects (Khundakar and Thomas, 2015).
Attempts were also made to reduce Ms A’s alprazolam dose under the supervision of her psychiatrist. As a result of even small reductions in the medication, Ms A experienced an increase in cycling between manic and depressive states. She developed severe suicidal ideations and affective lability. When describing her symptoms, Ms A said, “I go from crying to yelling in a matter of seconds.” It was felt that the patient was too fragile psychiatrically to attempt further adjustments to her psychopharmacologic regimen.
A disease management visit was scheduled with the Banner Alzheimer’s Institute Family and Community Services team to discuss nonpharmacologic aspects of care. It was highly recommended that stressors within the home be reduced, including consideration of a higher level of care such as an assisted living community for the patient’s mother, who continued to live with the patient and her partner. The mother’s increased needs and demands were a trigger for Ms A’s periods of distress and agitation and resulted in a greater tendency toward withdrawal into her bedroom.
Close monitoring of suicidal ideations was also recommended. Ms A had expressed that she would become actively suicidal if given the diagnosis of a neurodegenerative disorder. A safety plan was formulated with Ms A’s partner regarding what to do should suicidal ideations worsen.
DISCUSSION
The symptoms of dementia and depression frequently overlap, creating a diagnostic conundrum even for seasoned clinicians. Depression can be a risk factor, a prodrome, or a consequence of dementia.
Late-life depression is frequently associated with cognitive impairment. Dementia, in turn, has been associated with an increased risk of depressive symptoms. Depressive symptoms have been reported in 30% to 50% of patients with Alzheimer’s dementia, up to 50% of patients with vascular dementia, and approximately 50% of patients with Lewy body dementia (Zubenko et al, 2003; Park et al, 2007; Ballard et al, 1999). These symptoms appear to be especially common in the prodromal phase of illness. Neurodegeneration affecting the integrity of the frontostriatal circuits and the amygdala and hippocampus appears to increase the risk of developing depressive symptoms (Sexton et al, 2013).
Research also indicates that the presence of chronic depression during the lifetime may be associated with an increased risk for developing dementia, particularly vascular dementia (Barnes et al, 2012). The risk of developing dementia later in life increases 2-fold in the presence of a positive history of depression at a younger age. In recurrent depression, the risk of developing dementia is estimated to increase 14% with each depressive episode (Dotson et al, 2010).
An overlapping pathophysiology may explain the association between depression and dementia. There is significant evidence indicating that vascular disease is the primary link between depression and dementia. Vascular changes in the frontostriatal brain regions have been linked to both depressive symptomatology and cognitive impairment (Alexopoulos et al, 1997).
Hypercortisolemia present in depressive disorders has been associated with reduced hippocampal volumes and has a direct impact on Aβ processing, particularly due to alterations at the level of the serotoninergic system (Caraci et al, 2010), that may lead to an increased disequilibrium of Aβ production or clearance. Accumulation of Aβ plaques represents a fundamental pathologic hallmark of Alzheimer’s disease.
Depression also has been linked with pathophysiologic changes leading to deleterious effects on neuronal structure, function, and plasticity. Reduced plasma brain-derived neurotrophic factor levels have been observed in patients with depression (Karege et al, 2005). Depression also has been associated with proinflammatory cytokine overexpression, which interferes with serotonin metabolism and thereby decreases both synaptic plasticity and hippocampal neurogenesis (Leonard, 2007). Furthermore, depressed patients appear to develop accelerated cellular aging. Those with severe and chronic major depressive disorder display shorter telomere length than nondepressed controls (Verhoeven et al, 2014).
The clinical presentation of patients with Alzheimer’s dementia with depression and those presenting with depression with co-occurring cognitive symptoms may be very similar. Both conditions may present with a progressive amnestic syndrome with appearance of other cognitive, behavioral, and neuropsychiatric changes. In such cases, a more in-depth neuropsychological evaluation can assist with differential diagnosis.
Alzheimer’s dementia will present with an amnestic syndrome of hippocampal type characterized on tests, such as reduced word-list learning over trials, despite cueing. Damage to the hippocampi affects the ability to store new information. Therefore, information cannot be retrieved even after facilitation procedures (Tounsi et al, 1999).
In depressive disorders, memory deficits are related to attentional difficulties that impair encoding and retrieval strategies rather than to genuine storage deficits. These patients may benefit from repeated exposure to the word list and retrieval cues (Fossati et al, 2004).
Clinical Points
- The symptoms of dementia and depression frequently overlap; depression can be a risk factor, a prodrome, or a consequence of dementia.
- Depression has been linked with pathological changes leading to deleterious effects on neuronal structure, function, and plasticity and may increase the risk of cognitive impairment.
- Neuropsychological evaluation can help differentiate between dementia and depression by identifying patterns of cognitive strengths and weaknesses.
FUNDING/SUPPORT
None.
DRUG NAMES
Alprazolam (Xanax, Niravam, and others), benztropine (Cogentin and others), bupropion (Wellbutrin and others), citalopram (Celexa and others), donepezil (Aricept and others), gabapentin (Neurontin, Gralise, and others), lamotrigine (Lamictal and others), lithium (Lithobid and others), memantine (Namenda), omeprazole (Prilosec and others), quetiapine (Seroquel and others), risperidone (Risperdal and others), rivastigmine (Exelon and others), tramadol (Ultram and others).
DISCLOSURE OF OFF-LABEL USAGE
The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Financial Disclosure
Dr Anna D. Burke has served as a consultant to Eli Lilly. Dr William J. Burke has served as a consultant to Eli Lilly, FORUM, Forest, and OptumLabs and on the data monitoring committee of Otsuka. Drs Riggs, Weidman, and Copeland have no personal affiliations or financial relationships with any commercial interest to disclose relative to the activity.
Disclaimer
The opinions expressed are those of the authors, not of Banner Health or Physicians Postgraduate Press.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
Alexopoulos GS, Meyers BS, Young RC, et al. ‘ Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915-922. PubMed doi:10.1001/archpsyc.1997.01830220033006
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth edition. Arlington, VA: American Psychiatric Association; 2013.
Ballard C, Holmes C, McKeith I, et al. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer’s disease. Am J Psychiatry. 1999;156(7):1039-1045. PubMed
Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69(5):493-498. PubMed doi:10.1001/archgenpsychiatry.2011.1481
Caraci F, Copani A, Nicoletti F, et al. Depression and Alzheimer’s disease: neurobiological links and common pharmacological targets. Eur J Pharmacol. 2010;626(1):64-71. PubMed doi:10.1016/j.ejphar.2009.10.022
Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75(1):27-34. PubMed doi:10.1212/WNL.0b013e3181e62124
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed doi:10.1016/0022-3956(75)90026-6
Fossati P, Harvey PO, Le Bastard G, et al. Verbal memory performance of patients with a first depressive episode and patients with unipolar and bipolar recurrent depression. J Psychiatr Res. 2004;38(2):137-144. PubMed doi:10.1016/j.jpsychires.2003.08.002
Karege F, Vaudan G, Schwald M, et al. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136(1-2):29-37. PubMed doi:10.1016/j.molbrainres.2004.12.020
Khundakar AA, Thomas AJ. Neuropathology of depression in Alzheimer’s disease: current knowledge and the potential for new treatments. J Alzheimers Dis. 2015;44(1):27-41. PubMed
Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32(10):1749-1756. PubMed doi:10.1007/s11064-007-9385-y
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. PubMed doi:10.1111/j.1532-5415.2005.53221.x
Park JH, Lee SB, Lee TJ, et al. Depression in vascular dementia is quantitatively and qualitatively different from depression in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;23(2):67-73. PubMed doi:10.1159/000097039
Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry. 2013;21(2):184-195. PubMed doi:10.1016/j.jagp.2012.10.019
Tounsi H, Deweer B, Ergis AM, et al. Sensitivity to semantic cuing: an index of episodic memory dysfunction in early Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13(1):38-46. PubMed doi:10.1097/00002093-199903000-00006
Verhoeven JE, Révész D, Epel ES, et al. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry. 2014;19(8):895-901. PubMed doi:10.1038/mp.2013.151
Zubenko GS, Zubenko WN, McPherson S, et al. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer’s disease. Am J Psychiatry. 2003;160(5):857-866. PubMed doi:10.1176/appi.ajp.160.5.857
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top