Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
Neuroleptic malignant syndrome (NMS) is a severe idiosyncratic and potentially life-threatening adverse effect of antipsychotic therapy characterized by fever, rigidity, altered mental status, creatine kinase (CK) elevation, and autonomic instability. Although the pathophysiology of NMS has not been clearly elucidated, and NMS is not generally considered dose dependent and can occur within therapeutic dosage ranges of antipsychotics, NMS has been associated with rapid titration, higher cumulative antipsychotic dosages, and parenteral administration routes. Here, we report the case of a patient who developed NMS after treatment with multiple antipsychotics, including an early administration of risperidone long-acting injection.
Neuroleptic Malignant Syndrome After Early Administration of Risperidone Long-Acting Injection
To the Editor: Neuroleptic malignant syndrome (NMS) is a severe idiosyncratic and potentially life-threatening adverse effect of antipsychotic therapy characterized by fever, rigidity, altered mental status, creatine kinase (CK) elevation, and autonomic instability.1,2 Although the pathophysiology of NMS has not been clearly elucidated, and NMS is not generally considered dose dependent and can occur within therapeutic dosage ranges of antipsychotics, NMS has been associated with rapid titration, higher cumulative antipsychotic dosages, and parenteral administration routes.3-6 Here, we report the case of a patient who developed NMS after treatment with multiple antipsychotics, including an early administration of risperidone long-acting injection.
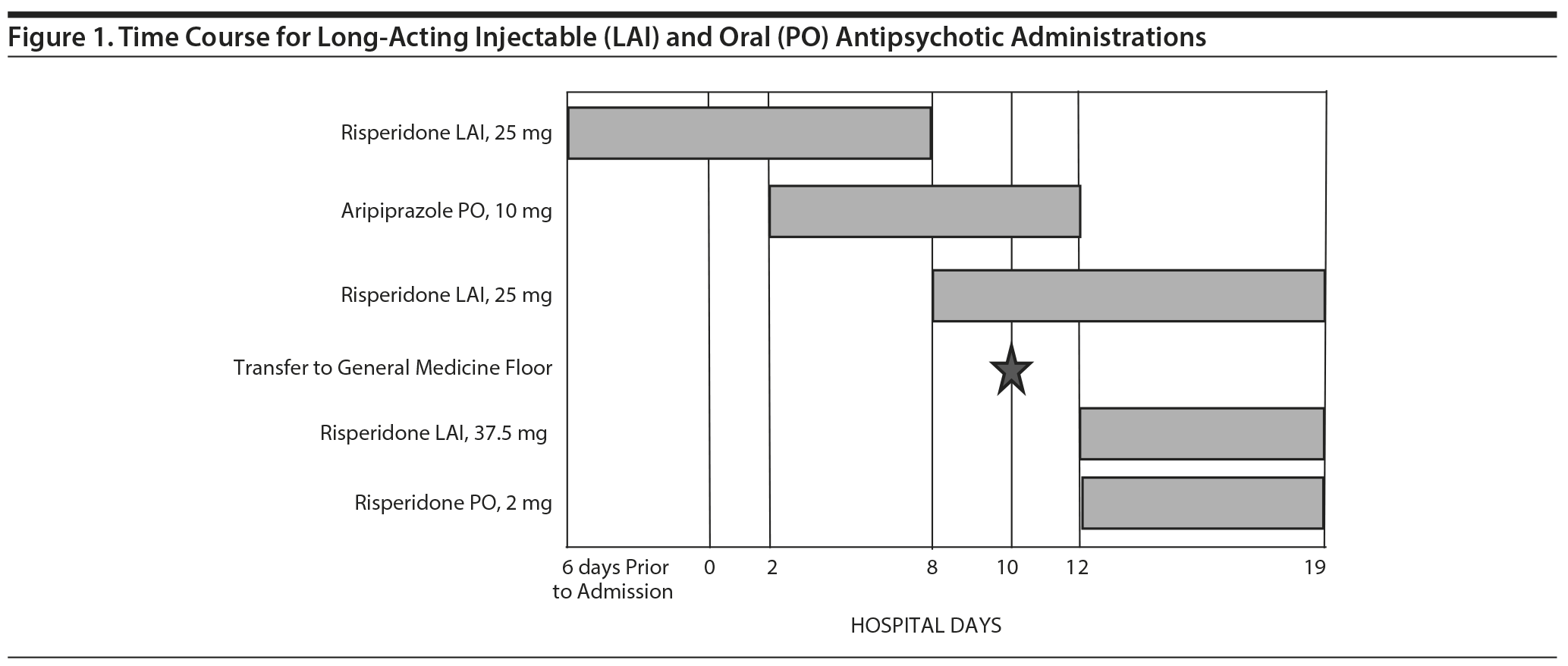
Case report. The patient is a 66-year-old white man with a history of recurrent major depressive disorder, severe, with psychotic features. He presented to the emergency department with altered mental status, insomnia, and agitated and aggressive behavior that started 2 weeks prior. He had been receiving risperidone long-acting injection 25 mg every 2 weeks for the past 4 years and received his last injection 6 days prior to admission. His other psychotropic medications included duloxetine 60 mg daily. On day 2 of this admission, the patient was started on aripiprazole 10 mg twice daily, which may have been due to his primary physician’s being unaware of previous risperidone injections. On day 8 of the admission, the patient continued to endorse worsening depression with hallucinations, and while being seen by a different psychiatrist who was covering in the absence of his primary psychiatrist, the patient received his next scheduled dose of risperidone 25-mg long-acting injection. On day 12 of the admission, the patient was seen by a third psychiatrist who was covering for his primary psychiatrist, and it was decided to discontinue the aripiprazole and prescribe an additional risperidone 37.5-mg long-acting injection and initiate risperidone 2 mg twice daily. The third psychiatrist was unaware of the previous risperidone injection, which was given on day 8 of the admission. Over the next few days, the patient exhibited fluctuating tachycardia, muscle rigidity, and diaphoresis. His psychomotor activity increased, and his cognitive function declined with reported psychotic and primitive behavior. He reported cold and clammy skin and extreme nausea with 3 to 4 episodes of vomiting and nonbloody diarrhea.
On day 10 of the admission, the patient was transferred to the general medicine floor due to hypotension and acute kidney injury, most likely secondary to volume depletion from his diarrhea, vomiting, and decreased fluid intake. He had a fluctuating temperature, ranging from 100.8°F to 101.1°F, which was treated with acetaminophen as needed. In addition, his blood pressure was also extremely labile, and he was observed performing constant "bicycle" movements of his lower extremities.
On day 17 of the admission, the patient continued to experience nausea, vomiting, and diarrhea and additionally had 1 episode of syncope. His CK level was elevated, ranging from 373 U/L to 783 U/L, which would eventually return to baseline. On day 19 of the admission, the psychiatric consult team noticed the patient had received an inappropriate dose of risperidone injection earlier in the admission and suggested his symptoms may be due to NMS. His daily risperidone dose was decreased and as-needed antipsychotics discontinued. He was given supportive care and slowly improved over time. Figure 1 provides the time course of antipsychotic administration during the patient’s hospital stay.
Neuroleptic malignant syndrome presents heterogeneously, and presentation when induced by second-generation antipsychotic drugs may be atypical with less motor symptoms.7 Altered mental status usually precedes systemic signs, and as this patient presented to the emergency department with altered mental status following an episode of metabolic encephalopathy, it is possible that onset was prior to the addition of neuroleptic agents, especially since NMS has not been proven to be dose dependent.2,8
In conclusion, NMS is a rare condition that is difficult to diagnose and often relies on time-cause relationships and careful assessment of antipsychotic load. While NMS and its risk factors are poorly understood, it is certain that careful monitoring when initiating or changing antipsychotic regimens can prevent or mitigate adverse effects.
References
1. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. PubMed CrossRef
2. Strawn JR, Keck PE, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. PubMed CrossRef
3. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
4. Langan J, Martin D, Shajahan P, et al. Antipsychotic dose escalation as a trigger for neuroleptic malignant syndrome (NMS): literature review and case series report. BMC Psychiatry. 2012;12(1):214. PubMed CrossRef
5. Keck PE Jr, Pope HG Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome: a case-control study. Arch Gen Psychiatry. 1989;46(10):914-918. PubMed CrossRef
6. Sachdev P, Mason C, Hadzi-Pavlovic D. Case-control study of neuroleptic malignant syndrome. Am J Psychiatry. 1997;154(8):1156-1158. PubMed CrossRef
7. Trollor JN, Chen X, Chitty K, et al. Comparison of neuroleptic malignant syndrome induced by first- and second-generation antipsychotics. Br J Psychiatry. 2012;201(1):52-56. PubMed CrossRef
8. Velamoor VR, Norman RM, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis. 1994;182(3):168-173. PubMed CrossRef
aDepartment of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, Texas
bDepartment of Pharmacy, Clear Lake Regional Medical Center, Houston, Texas
cDepartment of Pharmacy, Memorial Hermann Health System, Houston, Texas
Potential conflicts of interest: None.
Funding/support: None.
Acknowledgments: The authors thank Marie A. DeWitt, MD (Oscar G. Johnson Veterans Affairs Medical Center, Iron Mountain, Michigan), and Robert Scott Johnson, MD, JD, LLM (San Francisco Psychiatry, San Francisco, California), for their assistance during the patient’s admission to our health care facility. Drs DeWitt and Johnson report no conflicts of interest related to the subject of this article.
Patient consent: Permission was obtained from the patient to present this case, and all information has been de-identified to protect anonymity.
Published online: November 30, 2017.
Prim Care Companion CNS Disord 2017;19(6):17l02125
https://doi.org/10.4088/PCC.17l02125
© Copyright 2017 Physicians Postgraduate Press, Inc.
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top