ABSTRACT
Objective: To compare cardiovascular risk factors in patients with epilepsy with those of non-epileptic neurologic patients to determine their association with antiepileptic drug therapy.
Methods: This observational study with a cross-sectional design was performed in a tertiary care hospital in the Eastern Province of Saudi Arabia from January to December 2018. A total of 110 patients with epilepsy were included in the study, along with 46 age- and sex-matched non-epileptic controls (approximate ratio of 2:1). Blood pressure reading (BP), anthropometric measurements, fasting blood sugar levels, and fasting lipid profiles were performed for all subjects.
Results: Raised non–high-density lipid cholesterol (nHDLC) was the most common cardiovascular risk in epileptic patients, with a frequency of 51% compared to 30.4% in controls (P = .019). Epileptic patients who were male (58.3%, 28/48, P = .012) and those aged < 35 years (47.3%, 26/55, P = .036) were more likely to have high nHDLC. Obesity was also common in epileptic patients with frequency of 49.1% (n = 54) versus 30.4% (n = 14) in controls (P = .032). Metabolic syndrome was present in 26.3% of epileptic patients versus 23.9% of controls (P = .749). Among the epileptic patients, of those with high nHDLC, 85.7% had satisfactory seizure control (P = .019).
Conclusions: Raised nHDLC and obesity but not metabolic syndrome appear to be highly prevalent in epileptic patients compared to those without epilepsy. Antiepileptic drugs alone may not play a role in developing high lipid levels. More studies are needed to determine the causes of higher risk factor profile in epileptic patients and their relationship with seizure control.
Prim Care Companion CNS Disord 2022;24(3):21m03057
To cite: Khuda IE, Nazish S, Zeeshan MA, et al. Non-HDL cholesterol, obesity, and metabolic syndrome in epileptic patients. Prim Care Companion CNS Disord. 2022;24(3):21m03057.
To share: https://doi.org/10.4088/PCC.21m03057
© 2022 Physicians Postgraduate Press, Inc.
aDepartment of Neurology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
*Corresponding author: Saima Nazish, MBBS, FEBN, FCPS, Department of Neurology, King Fahd Hospital of University, Imam Abdulrahman Bin Faisal University, Khobar, Eastern Province, Saudi Arabia 31952 ([email protected]).
Cardiovascular risk factors, particularly metabolic syndrome, obesity, and lipid abnormalities, have been reported to be common among epileptic patients.1,2 These risk factors, however, are prevalent in the general population as well.3,4 Furthermore vascular risk factor profiles, especially that of metabolic syndrome, may vary in different geographic areas and among races within the same country.5 Therefore, evidence to prove a higher prevalence of vascular risk factors among epileptic patients is credible when there is a simultaneous control population from the same geographic area.
Metabolic syndrome is a term used when a few specific interrelated cardiovascular risk factors cluster in an individual that results in a 2-fold increased risk of cardiovascular disease.6 We tested the hypothesis that vascular risk factors may be more common in patients with epilepsy by comparing them with age- and sex-matched non-epileptic controls, all of whom (epileptics and controls) were residing in the Eastern Province of Saudi Arabia. The risk factors that we tested were metabolic syndrome, obesity (high body mass index [BMI]), and non–high-density lipid cholesterol (nHDLC), which is considered a more reliable evaluation for blood cholesterol to determine cardiovascular risk.7,8 It includes all the cholesterol types that are atherogenic, in a lipoprotein particle, which are low-density lipoprotein (LDL), intermediate-density lipoprotein, and very low-density lipoprotein. We also evaluated metabolic syndrome, obesity, and nHDLC in a secondary analysis that involved epileptic patients only to determine their clinical associations.
METHODS
This was an observational study with a cross-sectional design. The data were collected prospectively. After applying inclusion and exclusion criteria, 110 epileptic patients and 46 non-epileptic controls were recruited for the study. The patient recruitment and data collection were carried out in the Department of Neurology outpatient clinics at the study center from January to December 2018. The study center is a tertiary care university hospital in the Eastern Province of Saudi Arabia. The research was started after ethical approval from the institutional review board. A predesigned informed consent form in both Arabic and English was used as per the guidelines of the study center. The research was explained to the subjects, and they were recruited if they agreed to participate by signing the consent form.
Sampling Criteria
Consecutive patients visiting epilepsy and general neurology clinics as scheduled appointments were evaluated for possible recruitment. The inclusion criteria for both epileptic and control patients were (1) age ≥ 20 years, (2) resident of the Eastern Province of Saudi Arabia for at least the last 3 years, (3) able to stand upright for anthropometric measurements, (4) signed the informed consent form, and (5) visited the hospital outpatient laboratory within 7 days of signing the consent form after 8 to 10 hours of fasting to submit their blood samples for relevant investigations. Additional inclusion criteria for epileptic patients were a diagnosis of epilepsy for at least 1 year and receiving antiepileptic drug (AED) therapy for at least the same duration (1 year). Controls were age- and sex-matched to the epileptic patients participating in the study. They were recruited according to a ratio of 1:2 (there was 1 control for every 2 epileptic patients) within 6 months of recruitment of epilepsy patients. Pregnant patients were excluded from the study. Additional exclusion criteria for epileptic patients were those who were noncompliant with the therapy, considered as such by the treating physician, and those with inadequate therapy (underdosed or subtherapeutic AED levels). Control subjects were excluded if they had a personal or family history of epilepsy or were using AEDs for non-epileptic conditions.
Procedure
Body height, weight, and abdominal circumference were measured in the outpatient clinic by trained nursing staff. Weight and height were measured while subjects stood on a stationary stadiometer with a built-in digital weighing scale (Marsden M-100 column scale) after taking off their shoes and not holding any heavy objects. Blood pressure was measured while in the sitting position at least 5 minutes after the subjects entered the clinic using a digital blood pressure monitor (Omron 907). Abdominal circumference was measured using an inelastic, flexible tape that was held snugly at a point halfway between the iliac crest and the lowest palpable rib on either side as the subjects stood comfortably with their arms to their side.
The study was carried out in the outpatient department of the study center with a paperless management system, and all the patient information is directly stored in the electronic health records. Other relevant details about the included subjects were thus extracted from electronic health records. The study investigators also interviewed the subjects in case any relevant information was missing from their records. All the necessary data were finally entered in a predesigned proforma, then used for data analysis.
Operational Definitions
Patients were identified as having metabolic syndrome according to Adult Treatment Panel III criteria,9 which requires the presence of any 3 of the following 5 cardiovascular risk factors: (1) elevated blood pressure (hypertension): ≥130/85 mm Hg (or drug treatment of hypertension); (2) elevated fasting triglycerides: ≥ 150 mg/dL (or drug treatment for elevated triglycerides); (3) reduced high HDL: < 40 mg/dL for males and < 50 mg/dL for females; (4) elevated fasting blood sugar: ≥ 100 mg/dL (or on drug treatment for elevated blood glucose); and (5) increased waist circumference/obesity: for men ≥ 92 cm, for women ≥ 87 cm. Note that the cutoff values used for identifying increased waist circumference in the context of metabolic syndrome diagnosis were validated for the Saudi population.10 nHDLC was considered high when its level was more than 130 mg/dL. The nHDLC was calculated by subtracting HDL cholesterol from total cholesterol. BMI was calculated by dividing the weight in kilograms by the square of height in meters. Obesity when evaluated separately from metabolic syndrome was defined as BMI ≥ 30 kg/m2.
Epilepsy was defined according to the International League Against Epilepsy11 practical definition with at least 1 year of duration from the onset of seizures. Satisfactory seizure control was considered when the patients being treated for epilepsy had more than 50% reduction in seizure frequency pre- and post-intervention. Unsatisfactory seizure control was considered for patients who were treated for epilepsy and received well-tolerated, appropriately chosen AED schedules (as monotherapies or in combination) yet did not achieve more than 50% reduction of seizure frequency pre- and post-intervention. This included drug-refractory cases, which were those patients who could not achieve sustained seizure freedom despite receiving at least 2 AED schedules.12
Statistical Analysis
Values of categorical variables were taken as proportions and percentages and were compared by using Pearson χ2 and Fisher exact test. Mean values were taken for continuous variables and compared by using independent sample T test. The sample was also analyzed after stratification of data by sex and age, with age 35 years used as the cutoff value in the latter case. P values < .05 were considered significant. For multivariate analysis, a binary regression analysis model was used to estimate odds ratios and corresponding 95% confidence intervals of variables found statistically significant on univariate analysis. All the data were entered and analyzed by SPSS version 21.0 (SPSS Inc, IBM, Armonk, New York).
RESULTS
Patient Characteristics
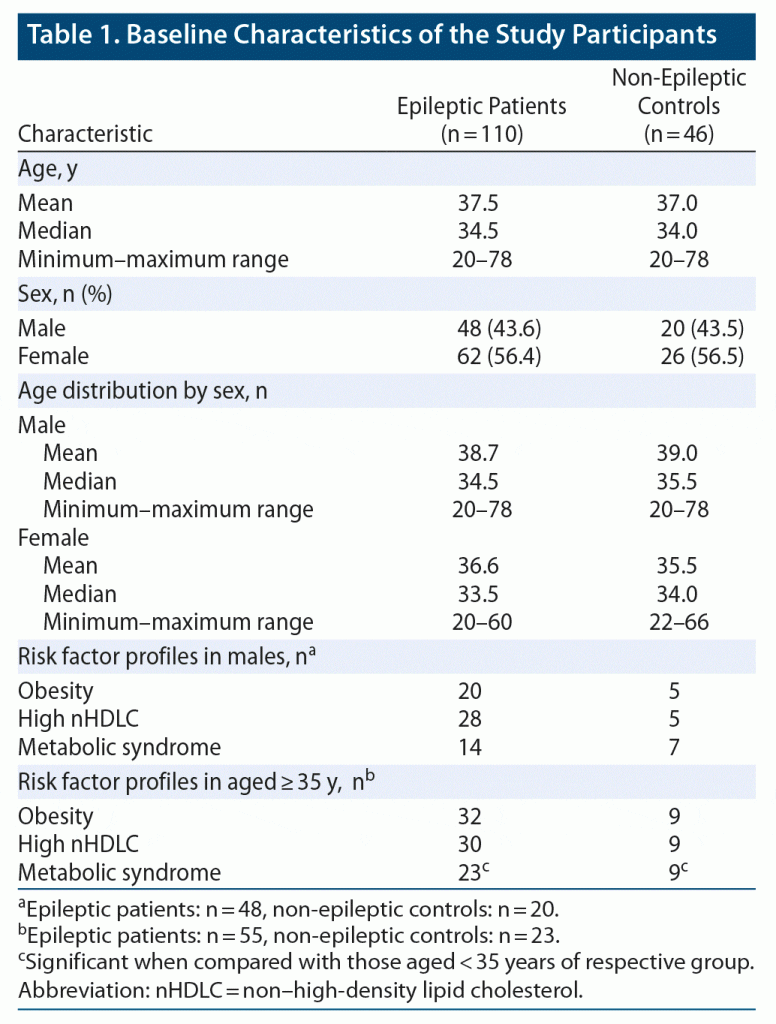
We recruited 110 epileptic patients and 46 age- and sex-matched non-epileptic controls after applying the inclusion and exclusion criteria. Table 1 shows the demographic characteristics of both epileptic patients and non-epileptic controls. The median age of epileptic patients and controls were 34.5 and 34.0 years, respectively, with a range (the minimum–maximum ages) in both groups of 20–78 years. Males comprised 43.6% of the epileptic patient group and 43.5% of the non-epileptic control group.
Univariate Analysis
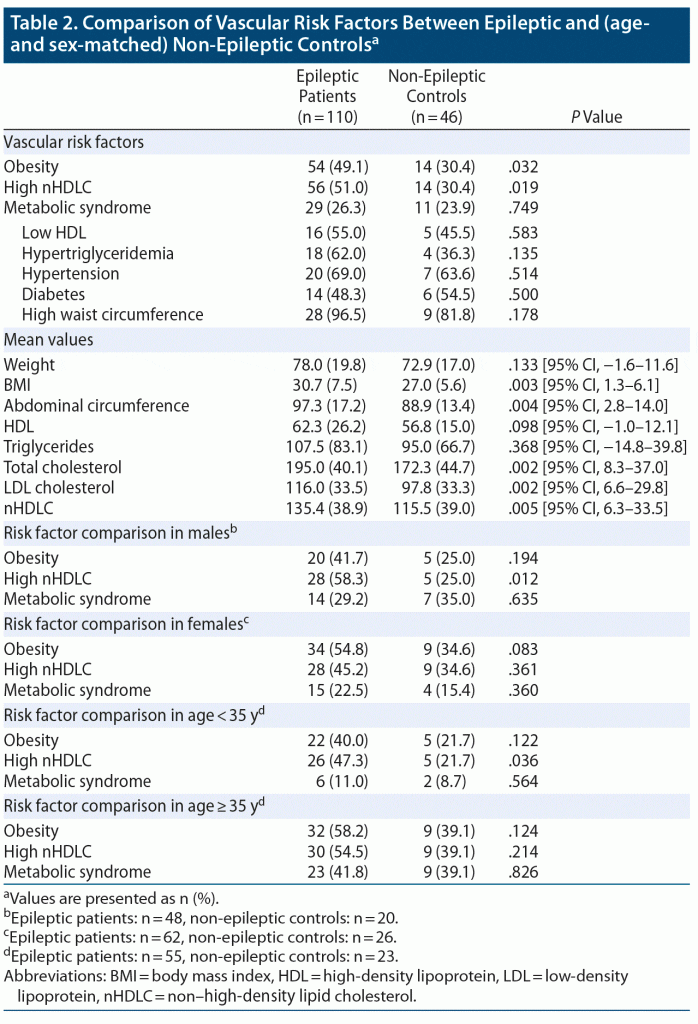
High nHDLC and obesity (high BMI) were significantly higher in epileptic patients compared to controls. High nHDLC was present in 50.9% (n = 56) of epileptic patients and 30.4% (n = 14) of controls (P = .019). Obesity (based on BMI) was found in 49.1% (n = 54) of epileptic patients and 30.4% (n = 14) of control subjects (P = .032). Metabolic syndrome, on the other hand, was found in 26.3% (n = 29) of epileptic patients and 23.9% (n = 11) of non-epileptic controls. This difference was not significant (P = .749). Regarding mean values of continuous variables, BMI, abdominal circumference, total cholesterol, LDL cholesterol, and nHDLC were significantly higher in epileptic patients compared to controls (Table 2).
Patients with metabolic syndrome. In the total sample (ie, both epileptic and non-epileptic patients), metabolic syndrome had a significantly higher frequency in older patients (aged ≥ 35 years age groups). There was no significant difference in the proportions of components of metabolic syndrome when epileptic and non-epileptic patients with metabolic syndrome were compared.
Analysis of Subgroups Based on Age
In the age-stratified analysis, epileptic patients aged < 35 years were found to have a statistically significant frequency of high nHDLC, which was found in 26 of 55 (47.3%) epileptic patients, compared to 5 of 23 (21.7%) age-matched controls (P = .036). There were no significant differences in the prevalence of other risk factors in the same age group. Furthermore, in patients aged ≥ 35 years, none of the evaluated risk factors, including metabolic syndrome, obesity, and high nHDLC, showed any significant differences.
Analysis of Subgroups Based on Sex
The sex-based stratified univariate analysis showed that high nHDLC was significantly more frequent in male epileptic patients compared to their sex-matched controls. Among male epileptic patients, the prevalence of high nHDLC was 58.3% (28/48) compared to 25% (5/20) in non-epileptic males (P = .012). Obesity, on the other hand, was not significantly different in frequency among males, with 20 of 48 (41.7%) epileptic patients versus 5 of 20 (21.5%) controls (P = .194) being obese. There were 14 of 48 male patients with metabolic syndrome compared to 7 of 20 non-epileptic controls (P = .635). There were no statistically significant differences in frequencies of high nHDLC, obesity, and metabolic syndrome between female epileptic patients and controls.
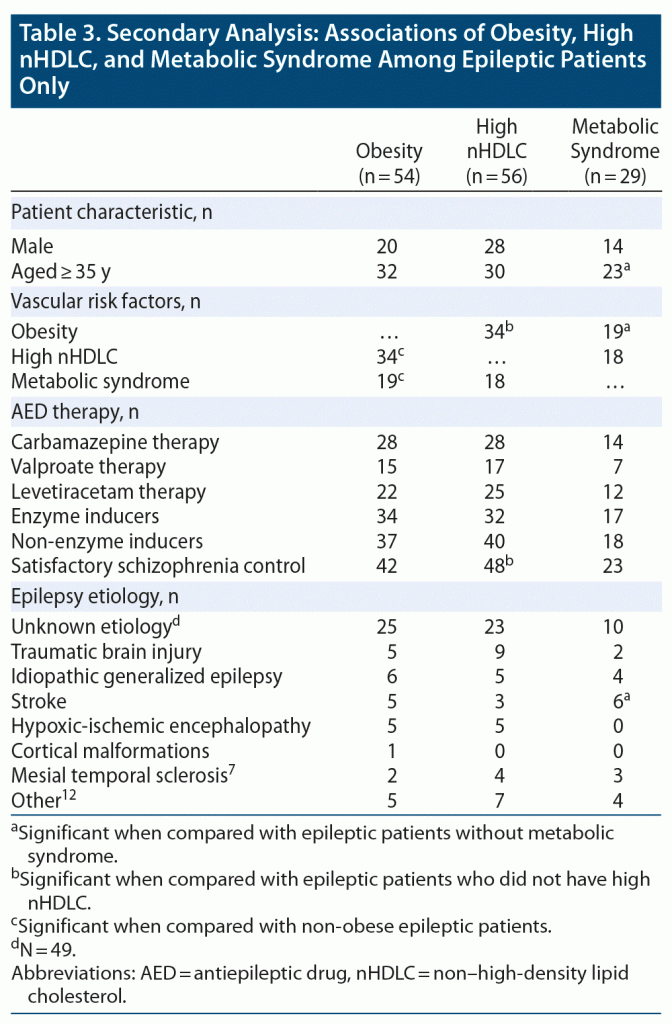
Interrelationships of risk factors in epileptic patients. Among the epileptic patients, obesity was strongly associated with high nHDLC (63%, 34/54, P =.003) and metabolic syndrome (35.2%, 19/54, P < .001). However, there was no significant association between high nHDLC and metabolic syndrome (Table 3).
Multivariate analysis. A multivariate analysis of categorical variables, by using a binary logistic regression model, found that high nHDLC was more significantly associated with epileptic patients (significant: P = .038, odds ratio [OR] = 2.190, 95% CI, 1.04–4.6) compared to obesity (significant: P = .064, OR = 2.017, 95% CI, 0.96–4.24).
Relationships between risk factors and other characteristics in epileptic patients. There was no association of obesity with any type of therapy or etiology of epilepsy. Most of the epileptic patients with high nHDLC had satisfactory seizure control (85.7%, 48/56, P = .019). However, there was no association of high nHDLC with any AED therapy. None of the etiologies of epilepsy were associated with high nHDLC among epileptic patients (Table 3).
There were 29 epileptic patients with metabolic syndrome, with significant associations with older age (aged ≥ 35 years, 23/29, P < .001), obesity (19/29, P = .039), and post-stroke epilepsy (6/29, P = .010). However, no significant association was found between epilepsy therapy and metabolic syndrome.
DISCUSSION
The key finding of our study is that the epileptic patients, irrespective of the epilepsy etiology and AED therapy, had a higher prevalence of raised nHDLC compared to non-epileptic controls. Epileptic patients with younger age and male sex were more likely to have high nHDLC. Obesity was also common in patients with epilepsy, especially in those who had high nHDLC or metabolic syndrome. The association of abnormal lipid profile and obesity in epileptic patients has not been convincingly established in the published literature. In a population-based study, Mintzer et al13 found a higher prevalence of hyperlipidemia in male adults with epilepsy compared to the general population. This was a database and prescription-based study,13 in which serum lipids were not measured for all the patients. Arya et al14 found a higher prevalence of obesity in adolescent patients newly diagnosed with childhood (onset) absence epilepsy. Janousek et al,15 however, did not find adult epileptic patients to be more obese compared to the general population. In both studies,14,15 there was a lack of direct comparison with the control population, and historical controls were used instead. Another study16 suggested an association of obesity with idiopathic generalized epilepsy and family history of epilepsy; however, it also lacked a comparison with non-epileptic controls.
We evaluated nHDLC, as it has shown to be a better marker of cardiovascular risk. Very few studies have measured nHDLC in epileptic patients. In a large number of epileptic patients, Yamamoto et al17 found a high level of nHDLC, especially in those taking carbamazepine and valproic acid. This study17 also lacked nHDLC levels in non-epileptic subjects for comparison. Our study also showed a significantly higher proportion of abnormally elevated nHDLC in younger epileptic patients compared to non-epileptic controls in the same age group. Elevated nHDLC along with obesity earlier in life, if not identified and corrected, may increase the likelihood of cardiovascular morbidity due to long-standing effects on body metabolism, thereby increasing atherosclerosis of blood vessels. This might explain the higher cardiac comorbidity in epileptic patients as shown by Zack and Luncheon18 in a large population-based study. Metabolic syndrome per se had a similar prevalence in epileptic patients and non-epileptic controls. However, given the higher prevalence of obesity and abnormally high nHDLC levels, higher cardiovascular risk in epileptic patients is still a concern.
Our secondary analysis, which involved only epileptic patients, showed that obesity was significantly associated with high nHDLC levels and metabolic syndrome. In our study, metabolic syndrome among epileptic patients was mostly found in older and obese people, but not in those with high nHDLC. Metabolic syndrome was also significantly associated with post-stroke epilepsy. This association, to the best of our knowledge, has not been shown in previous studies; hence, we recommend more observational studies in this context to confirm or refute this finding.
Interestingly high nHDLC was significantly associated with satisfactory seizure control in our patients. Satisfactory seizure control with obesity was shown previously by Arya et al14; however, serum lipids (that correlate with obesity) were not measured in that study. Hyperlipidemic effects of the ketogenic diet, which has a well-documented seizure-suppressing effect, have also been documented by Cervenka et al.19 However, the effects of hyperlipidemia on seizure control needs to be explored further by more studies with more precise case definitions of seizure control.
Metabolic syndrome, dyslipidemia, and obesity can also result from AED use (especially with carbamazepine and valproate). However, this association has not been consistently shown in the literature. Moses et al20 could not find a relationship between raised BMI or altered blood lipids in epileptic patients with intellectual disabilities treated with valproate. Our study also could not show an association of nHDLC levels, obesity, or metabolic syndrome with the tested AEDs (carbamazepine, levetiracetam, and valproic acid). When AEDs were grouped according to their mechanism of action (ie, enzyme-inducing vs non-inducing AEDs), we found no significant results when comparing the risk factors in these groups. This lack of association could be due to a smaller sample size that may have resulted in insufficient power.
Cardiovascular risk factors including obesity and dyslipidemia are also related to the individual’s lifestyle and physical activity.21 The Saudi population, in general, has a low level of physical activity as well, which may be a factor contributing to high levels of obesity.22 Hence, it seems more likely that many factors may play a role in determining the metabolic status and body weight of epileptic patients. Therefore, management of obesity and dyslipidemia should be individualized according to the patients’ characteristics. A deeper understanding is required to ascertain the reasons for abnormal blood lipids and obesity. Perhaps a lack of physical activity or inappropriate dietary habits may play an essential role in some obese and hyperlipidemic epileptic patients. In that case, psychoeducation (with emphasis on physical exercises) or professional advice on weight loss strategies may be employed before trying an unfruitful or potentially dangerous switch of AEDs that otherwise control the seizures.
One of the limitations of our study is that it was carried out at a tertiary care center with a comparison group from a general neurology outpatient clinic; thus, our study subjects were a selected population, which may have resulted in a selection bias. Furthermore, since we do not have the baseline vascular risk factor profile of epileptic patients before the onset of epilepsy, we cannot make any causal associations from our findings.
CONCLUSION
High nHDLC and obesity are more frequent among epileptic patients, while those of younger age and male sex seem to have a high predisposition to have raised nHDLC. AEDs alone may not play a role in the development of high lipid levels, obesity, or metabolic syndrome among epileptic patients, and other contributing factors should also be identified in epileptic individuals.
Submitted: June 20, 2021; accepted September 28, 2021.
Published online: June 7, 2022.
Relevant financial relationships: None.
Funding/support: This study was funded by Imam Abdulrahman Bin Faisal University, Deanship of Scientific research (vice presidency for graduate studies) through funding opportunity of UOD-2017 newly recruited faculty members program, Dammam, Saudi Arabia.
Role of the sponsor: The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; or preparation, review, or approval of the manuscript.
Acknowledgments: This study made use of the computational resources and technical services of the Scientific and High Performance Computing Center at Imam Abdulrahman Bin Faisal University, Saudi Arabia. The authors acknowledge and express their sincere gratitude to Khurum H. Khan, MD (University College London Hospitals, NHS Foundation Trust, London, England) for revising the manuscript and providing valuable suggestions, as well as the following undergraduate medical students from the College of Medicine, Imam Abdulrahman Bin Faisal University for their contribution in data collection and hard work in carrying out this research project: Modhi Alajmi, MBBS; Amira Alolyani, MBBS; Sara Al Arhain, MBBS; Ashwaq Alanazi, MBBS; and Sara Al Mohammed, MBBS. The acknowledged individuals report no conflicts of interest related to the subject of this article.
Clinical Points
- High non–high-density lipid cholesterol (nHDLC; dyslipidemia) and obesity are prevalent in epileptic patients; younger and male epileptic patients are more likely to have high nHDLC levels.
- Dyslipidemia as well as obesity earlier in life, if not identified and corrected, may raise the likelihood of cardiovascular morbidity due to long-standing effects on metabolism, increasing atherosclerosis of blood vessels.
- Among the several possible causes for dyslipidemia and obesity in epilepsy, lack of exercise, behavioral problems, and medications are the main factors to be addressed.
References (22)

- Harnod T, Chen HJ, Li TC, et al. A high risk of hyperlipidemia in epilepsy patients: a nationwide population-based cohort study. Ann Epidemiol. 2014;24(12):910–914. PubMed CrossRef
- Vivanco-Hidalgo RM, Gomez A, Moreira A, et al. Prevalence of cardiovascular risk factors in people with epilepsy. Brain Behav. 2016;7(2):e00618. PubMed CrossRef
- Al-Rubeaan K, Bawazeer N, Al Farsi Y, et al. Prevalence of metabolic syndrome in Saudi Arabia: a cross sectional study. BMC Endocr Disord. 2018;18(1):16. PubMed CrossRef
- Yusuf S, Hawken S, Ounpuu S, et al; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. PubMed CrossRef
- Gurka MJ, Filipp SL, DeBoer MD. Geographical variation in the prevalence of obesity, metabolic syndrome, and diabetes among US adults. Nutr Diabetes. 2018;8(1):14. PubMed CrossRef
- Nair SS, Harikrishnan S, Sarma PS, et al. Metabolic syndrome in young adults with epilepsy. Seizure. 2016;37:61–64. PubMed CrossRef
- Arsenault BJ, Boekholdt SM, Kastelein JJ. Lipid parameters for measuring risk of cardiovascular disease. Nat Rev Cardiol. 2011;8(4):197–206. PubMed CrossRef
- Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 2012;307(12):1302–1309. PubMed CrossRef
- Grundy SM, Cleeman JI, Daniels SR, et al; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. PubMed CrossRef
- Al-Rubean K, Youssef AM, AlFarsi Y, et al. Anthropometric cutoff values for predicting metabolic syndrome in a Saudi community: from the SAUDI-DM study. Ann Saudi Med. 2017;37(1):21–30. PubMed CrossRef
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. PubMed CrossRef
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. PubMed CrossRef
- Mintzer S, Yi M, Hegarty S, et al. Hyperlipidemia in patients newly treated with anticonvulsants: a population study. Epilepsia. 2020;61(2):259–266. PubMed CrossRef
- Arya R, Gillespie CW, Cnaan A, et al; Childhood Absence Epilepsy Study Group. Obesity and overweight as CAE comorbidities and differential drug response modifiers. Neurology. 2016;86(17):1613–1621. PubMed CrossRef
- Janousek J, Barber A, Goldman L, et al. Obesity in adults with epilepsy. Epilepsy Behav. 2013;28(3):391–394. PubMed CrossRef
- Ladino LD, Hernández-Ronquillo L, Téllez-Zenteno JF. Obesity and its association with generalised epilepsy, idiopathic syndrome, and family history of epilepsy. Epileptic Disord. 2014;16(3):343–353. PubMed CrossRef
- Yamamoto Y, Terada K, Takahashi Y, et al. Influence of antiepileptic drugs on serum lipid levels in adult epilepsy patients. Epilepsy Res. 2016;127:101–106. PubMed CrossRef
- Zack M, Luncheon C. Adults with an epilepsy history, notably those 45–64 years old or at the lowest income levels, more often report heart disease than adults without an epilepsy history. Epilepsy Behav. 2018;86:208–210. PubMed CrossRef
- Cervenka MC, Patton K, Eloyan A, et al. The impact of the modified Atkins diet on lipid profiles in adults with epilepsy. Nutr Neurosci. 2016;19(3):131–137. PubMed CrossRef
- Moses L, Katz N, Weizman A. Impact of epilepsy and antiepileptic medications on the metabolic profile in adults with autism spectrum disorder and intellectual disabilities. Int Clin Psychopharmacol. 2015;30(6):351–355. PubMed CrossRef
- Martinez-Gomez D, Guallar-Castillon P, Higueras-Fresnillo S, et al. A healthy lifestyle attenuates the effect of polypharmacy on total and cardiovascular mortality: a national prospective cohort study. Sci Rep. 2018;8(1):12615. PubMed CrossRef
- Al-Hazzaa HM. Physical inactivity in Saudi Arabia revisited: a systematic review of inactivity prevalence and perceived barriers to active living. Int J Health Sci (Qassim). 2018;12(6):50–64. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top