Objective: Here, we examine rates of intracranial tumor diagnoses in patients with and without comorbid psychiatric diagnoses to better understand how psychiatric disease may alter risk profiles for brain tumor diagnosis.
Methods: We used a longitudinal version of the California Office of Statewide Health Planning and Development (OSHPD) database, which includes all inpatient admissions in California from 1995 to 2010. We examined patients with confirmed hospital admissions from 1997 to 2004. Patients with an intracranial tumor or psychiatric diagnosis on their first hospital admission were excluded. The primary outcome of interest was the diagnosis of intracranial tumor on any subsequent hospitalization within 5 years. Risk of tumor diagnosis was determined via Cox proportional hazard models adjusted for age, gender, race/ethnicity, and comorbidity burden. Subset analyses were performed for various tumor types.
Results: The risk for diagnosis of an intracranial tumor within 5 years, as determined by the hazard ratio, was 1.61 (95% CI, 1.28-2.04) for bipolar, 1.59 (95% CI, 1.41-1.72) for anxious, and 1.34 (95% CI, 1.25-1.43) for depressed cohorts relative to controls. More specifically, the risk for diagnosis of a primary benign neoplasm was elevated in depressed patients, while the risk for diagnosis of a meningioma was elevated in depressed, anxious, and bipolar disorder patients.
Conclusions:Patients admitted with certain psychiatric diagnoses appear more likely to be readmitted within 5 years with specific types of intracranial tumor diagnoses. The association between certain psychiatric diagnoses and subsequent brain tumor diagnosis most likely reflects the long-held belief that slow-growing tumors may first present as psychiatric symptoms before being diagnosed. Primary care physicians should consider the possibility of an underlying intracranial tumor in patients with new psychiatric diagnoses.
Objective: Here, we examine rates of intracranial tumor diagnoses in patients with and without comorbid psychiatric diagnoses to better understand how psychiatric disease may alter risk profiles for brain tumor diagnosis.
Methods: We used a longitudinal version of the California Office of Statewide Health Planning and Development (OSHPD) database, which includes all inpatient admissions in California from 1995 to 2010. We examined patients with confirmed hospital admissions from 1997 to 2004. Patients with an intracranial tumor or psychiatric diagnosis on their first hospital admission were excluded. The primary outcome of interest was the diagnosis of intracranial tumor on any subsequent hospitalization within 5 years. Risk of tumor diagnosis was determined via Cox proportional hazard models adjusted for age, gender, race/ethnicity, and comorbidity burden. Subset analyses were performed for various tumor types.
Results: The risk for diagnosis of an intracranial tumor within 5 years, as determined by the hazard ratio, was 1.61 (95% CI, 1.28-2.04) for bipolar, 1.59 (95% CI, 1.41-1.72) for anxious, and 1.34 (95% CI, 1.25-1.43) for depressed cohorts relative to controls. More specifically, the risk for diagnosis of a primary benign neoplasm was elevated in depressed patients, while the risk for diagnosis of a meningioma was elevated in depressed, anxious, and bipolar disorder patients.
Conclusions:Patients admitted with certain psychiatric diagnoses appear more likely to be readmitted within 5 years with specific types of intracranial tumor diagnoses. The association between certain psychiatric diagnoses and subsequent brain tumor diagnosis most likely reflects the long-held belief that slow-growing tumors may first present as psychiatric symptoms before being diagnosed. Primary care physicians should consider the possibility of an underlying intracranial tumor in patients with new psychiatric diagnoses.
Prim Care Companion CNS Disord 2016;18(6):doi:10.4088/PCC.16m02028
© Copyright 2016 Physicians Postgraduate Press, Inc.
aSchool of Medicine, University of California, San Diego
bDepartment of Neurosurgery, University of California, San Diego
cDepartment of Psychiatry, University of California, San Diego
‡Equal contributions as first authors.
*Corresponding author: Bob S. Carter, MD, PhD, Department of Neurosurgery, University of California, San Diego, CA ([email protected]).
For patients diagnosed with brain tumors, coping with the uncertainty surrounding treatment, prognosis, and personal implications can create significant psychological stress.1 Not surprisingly, therefore, patients diagnosed with brain tumors are often at higher risk for being diagnosed with various psychiatric disorders.2,3 In this context, the association between brain tumors and psychiatric disease is well established, if only for its intuitive logic. The extent of association between preexisting psychiatric diagnoses and subsequent brain tumors, however, is less well understood. In this study, we investigate this association in greater detail.
The relationship between preexisting psychiatric diagnoses and brain tumors can generally be considered in 1 of 2 major contexts. The first suggests that psychiatric disease may alter one’s risk for developing a brain tumor through a variety of potential mediators known to associate with psychiatric patients. These mediators include differences in behavioral and lifestyle trends,4 fluctuations in immunologic surveillance resulting from psychological stress,5 changes in circulating hormones,6,7 or even epigenetic changes observed in psychiatrically distressed individuals.8
Alternatively, the second context suggests a reversed relationship in which psychiatric disease may simply represent the earliest manifestation of an underlying brain tumor,9-11 particularly given that psychiatric symptoms have been shown to correlate with specific tumor locations within the brain.9,12 This theory has been applied mainly to slow-growing or diffuse tumors, which have the potential to produce isolated psychiatric symptoms long before becoming overtly symptomatic.13,14 In light of these 2 opposing viewpoints, this study aims to further elucidate this association by using a large-scale administrative database to investigate the long-term risk of intracranial tumor diagnosis for patients with and without psychiatric disease.
METHODS
Data Set and Inclusion/Exclusion Criteria
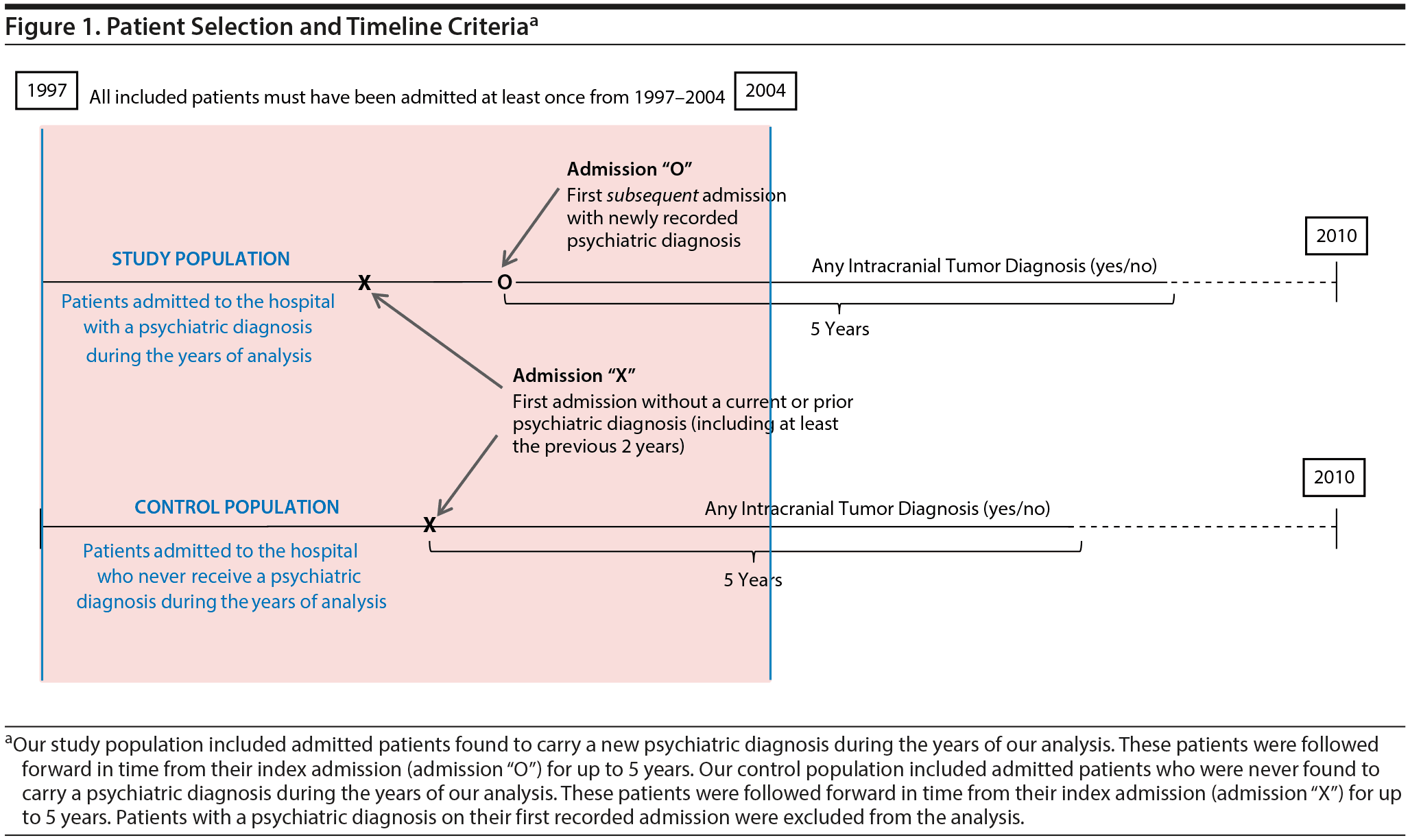
This historical prospective cohort study was conducted using the longitudinal California Office of Statewide Health Planning and Development (OSHPD) database (1995-2010), which includes a record of all inpatient admissions in the state of California at participating hospitals during those years. Figure 1 illustrates our patient selection criteria and methods. All patients with a recorded hospital admission in the state of California from 1997 to 2004 (inclusive) were included to ensure a minimum of 5 years of follow-up for all patients. For each patient identified and included, admission data were collected from all other hospital admissions between 1995 and 2010. Patients were then excluded if their earliest recorded admission occurred prior to January 1, 1997, in order to assure that all included patients had at least 2 years with no prior hospitalizations. Patients were also excluded if their first recorded admission included a diagnosis of intracranial tumor or of depression, anxiety, bipolar disorder, or schizophrenia (determined using Internal Classification of Diseases, 9th edition, Clinical Modification [ICD-9-CM] codes; see Supplemental Data for all codes)to avoid including patients with unknown prior disease duration or with an altered intracranial tumor risk profile. Finally, patients were excluded if diagnosed with multiple intracranial tumor diagnoses, secondary intracranial metastases, or an intracranial tumor of “uncertain behavior,” as defined by ICD-9-CM codes (see Supplemental Data) to avoid tumor misclassification or uncertainty in diagnosis. Eligible patients were followed forward in time from their first admission of interest—termed the index admission—until they reached the study endpoint (5 years of follow-up), death, or the outcome of interest.

- The 5-year risk for intracranial tumor diagnosis is higher for patients diagnosed with certain psychiatric illnesses.
- The association between certain psychiatric diagnoses and subsequent brain tumor diagnosis most likely reflects the long-held belief that slow-growing tumors may first present as psychiatric symptoms before being diagnosed.
- Primary care physicians should consider the possibility of an underlying intracranial tumor in patients with new psychiatric diagnoses.
The Index Admission
Figure 1 includes a graphical representation of the index admission, which effectively establishes a timepoint “zero” for each patient cohort. For patients found to carry a psychiatric diagnosis (ie, depression, anxiety, bipolar disorder, or schizophrenia), the index admission was set as the first psychiatric-comorbid admission (admission “O” in Figure 1). For patients who were never found to carry a psychiatric diagnosis of interest, the index admission was set simply as the first recorded admission (admission “X” in Figure 1). The index admission, therefore, differed for patients with and without psychiatric diagnoses so as to follow patients from their first available admission of interest.
Depression, Anxiety, Bipolar Disorder, and Schizophrenia
The presence of a psychiatric diagnosis—specifically depression, anxiety, bipolar disorder, or schizophrenia—was determined using ICD-9-CM codes from recorded admission data. Patients were considered to be depressed, anxious, bipolar, or schizophrenic depending on their respective diagnosis codes during the years of our analysis. However, those patients who received a psychiatric diagnosis during or after an admission with an intracranial tumor diagnosis were reclassified as having no psychiatric disease (ie, control population), in order to investigate the risk of tumor diagnosis for patients with preexisting psychiatric disease only.
Outcomes
Our primary outcome of interest was the diagnosis of an intracranial tumor on a hospital admission within 5 years of the index admission. Our secondary outcome of interest was the diagnosis of a specific intracranial tumor subtype within 5 years of the index admission, which included primary malignant neoplasm of the brain, primary benign neoplasm of the brain, cranial nerve tumor, or meningioma.
Covariates
Multivariable analysis included the covariates of patient age, gender, race/ethnicity, and comorbidity burden (as determined by the Charlson Comorbidity Index) during the index admission. Patients who were never found to carry the psychiatric diagnosis in question (ie, depression, anxiety, bipolar disorder, or schizophrenia) were set as the reference group for all hazards modeling.
Statistical Analysis
The risk of intracranial tumor diagnosis in each patient cohort was assessed using a Cox proportional hazards model adjusting for the previously mentioned covariates. Cox proportional hazards models were run separately and independently for each psychiatric cohort, such that the risk could be calculated for each individual psychiatric diagnosis irrespective of the presence or absence of others. Given that this study focused on risk profiles for individual psychiatric diagnoses, those patients carrying more than 1 psychiatric diagnosis were not classified separately; they were instead included in the analyses multiple times—once for each of their psychiatric diagnoses. For example, a patient with depression and anxiety diagnoses would be classified as “depressed” for the purposes of investigating depression and “anxious” for the purposes of investigating anxiety but not as both “depressed and anxious” for the purposes of our analysis.
Subset Analyses
To determine the relative contributions of different tumor subtypes to our observed findings, we performed a series of subset analyses to determine the risk of receiving a diagnosis of specific types of intracranial tumor, namely primary malignant neoplasms, primary benign neoplasms, cranial nerve tumors, and meningioma for each psychiatric cohort of interest. For meningioma specifically—a tumor for which female gender is a known risk factor15—we performed a gender-stratified analysis to determine the relative effects of each psychiatric diagnosis for each gender cohort.
Data Analysis Tools
Commercially available software (STATA SE Version 11.2, Stata Corp LP, College Station, Texas) was used to perform the statistical analysis. Statistical significance was defined as P < .05 for our primary analysis and P < .05/4 for all subset analyses so as to adjust for multiple comparisons; all tests were 2-sided.
RESULTS
Baseline Demographics
A total of 6,996,978 patients were included in our primary analysis. A total of 496,987 patients (7.1%) were classified as depressed; 210,382 (3.0%) as anxious; 54,295 (0.8%) as bipolar; and 40,666 (0.6%) as schizophrenic during the years of our analysis. Of all included patients, 14,544 received a diagnosis of intracranial tumor, which was recorded on a hospitalization within 5 years of their index admission. Of these intracranial tumors, 4,881 were classified as primary malignant; 714 were primary benign; 1,195 were cranial nerve neoplasms; and 7,744 were meningiomas. Baseline characteristics for all patients, as well as for each psychiatric cohort, are available in Table 1.
Adjusted Multivariable Analyses
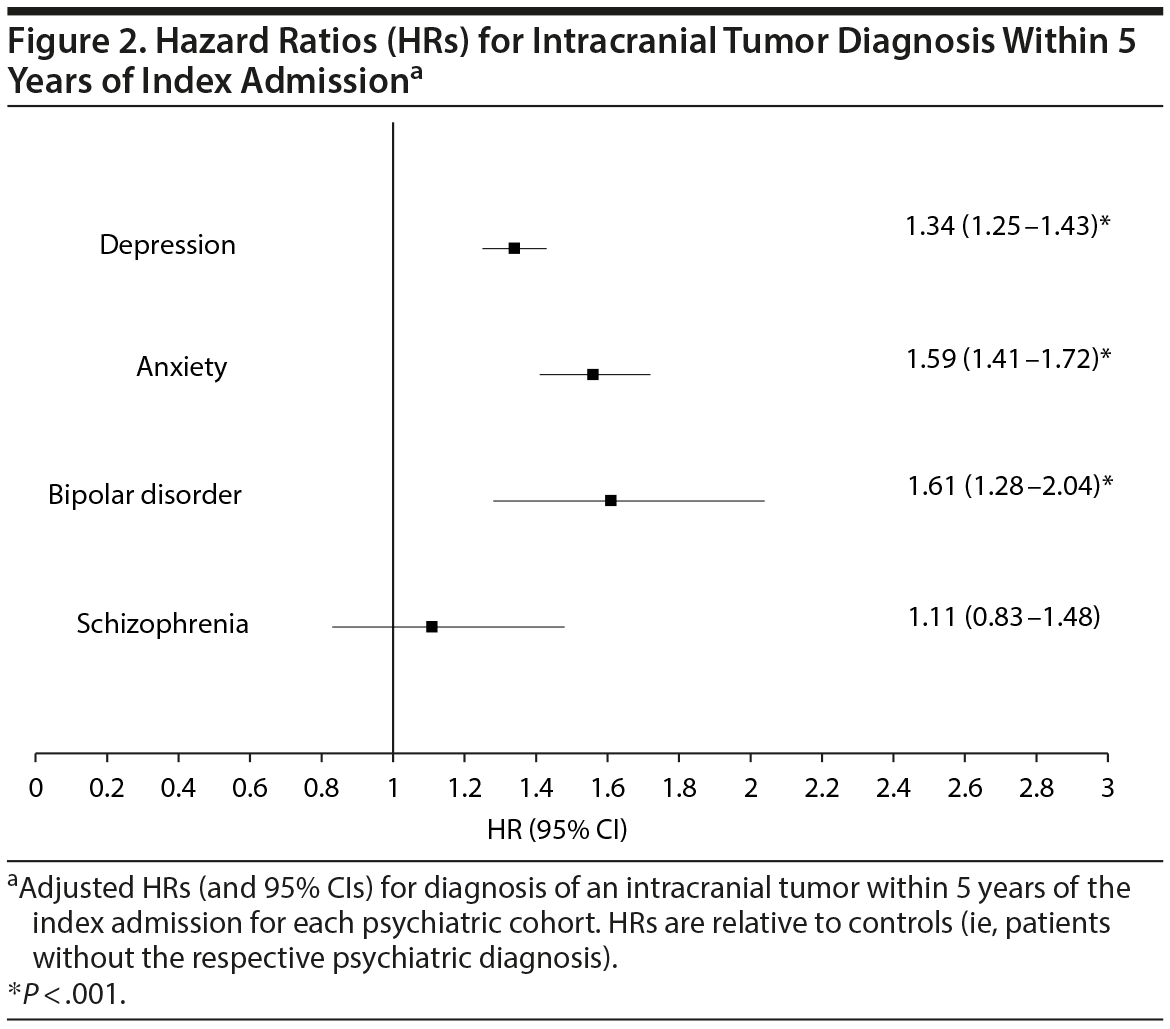
Cox proportional hazards modeling, adjusting for patient age, gender, race/ethnicity, and comorbidity burden, revealed that the hazard ratio (HR) for being diagnosed with an intracranial tumor within 5 years of the index admission was highest for patients with a preexisting diagnosis of bipolar disorder (HR = 1.61; 95% CI, 1.28-2.04 relative to nonbipolar patients), followed by anxiety (HR = 1.59; 95% CI, 1.41-1.72 relative to nonanxious patients), and then depression (HR = 1.34; 95% CI, 1.25-1.43 relative to no depression). Patients with preexisting diagnosis of schizophrenia did not have a significantly different HR (HR = 1.11; 95% CI, 0.83-1.48) relative to no schizophrenia for intracranial tumor diagnosis. HR data are available for review in Table 2 and Figure 2.
Subset Analyses
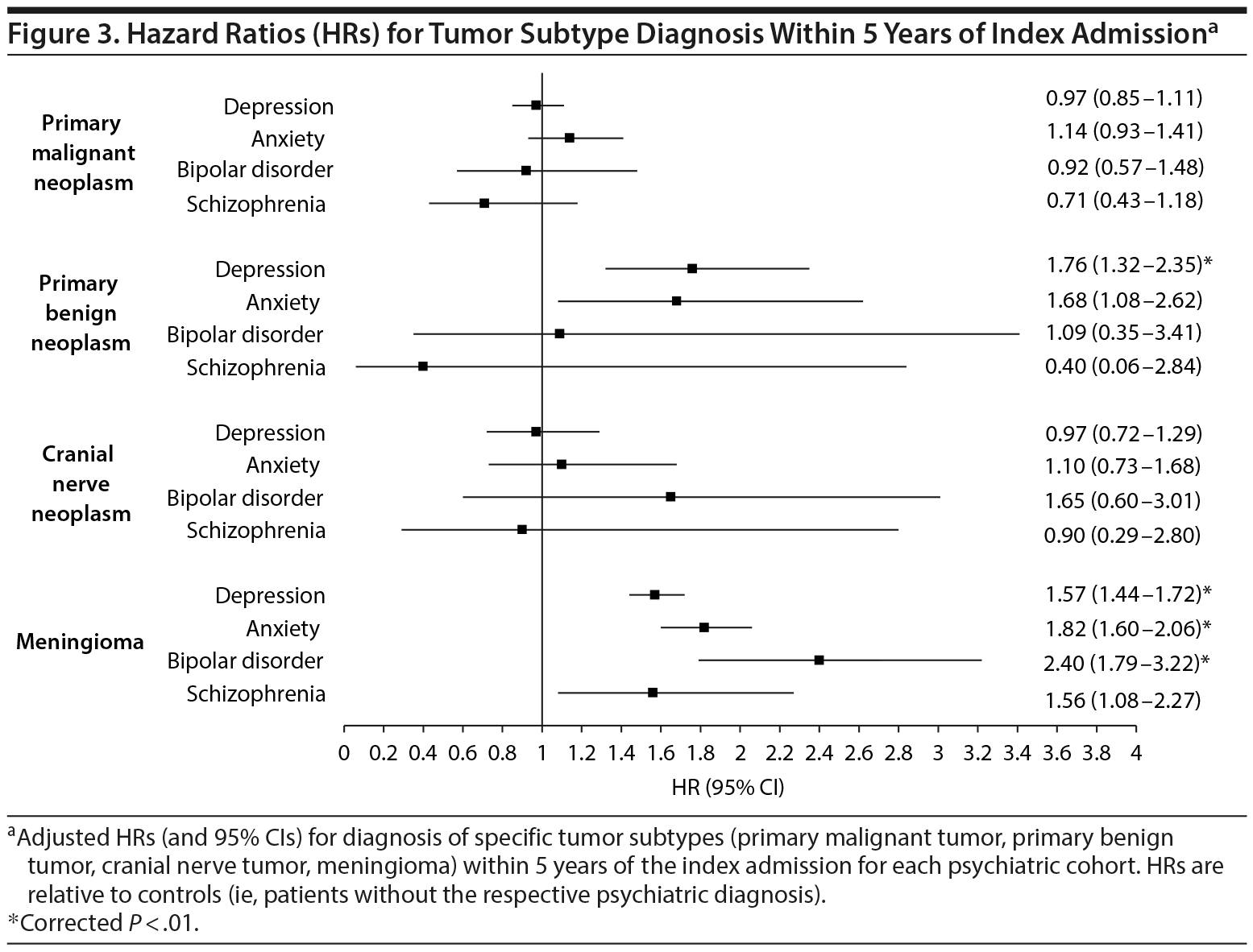
After adjustment for multiple comparisons post hoc, the risk of primary malignant neoplasm or of cranial nerve tumor diagnosis was not significantly different for any psychiatric cohort relative to their nonpsychiatric references. The risk for primary benign neoplasm, however, was significantly higher for patients with a diagnosis of depression, relative to patients without a depression diagnosis (P < .01). Finally, the risk for meningioma was significantly higher for depressed, anxious, and bipolar cohorts relative to patients without depression, anxiety, or bipolar disorder, respectively (P < .01). The results for these subset analyses are displayed in Figure 3.
Meningioma and Gender Stratification
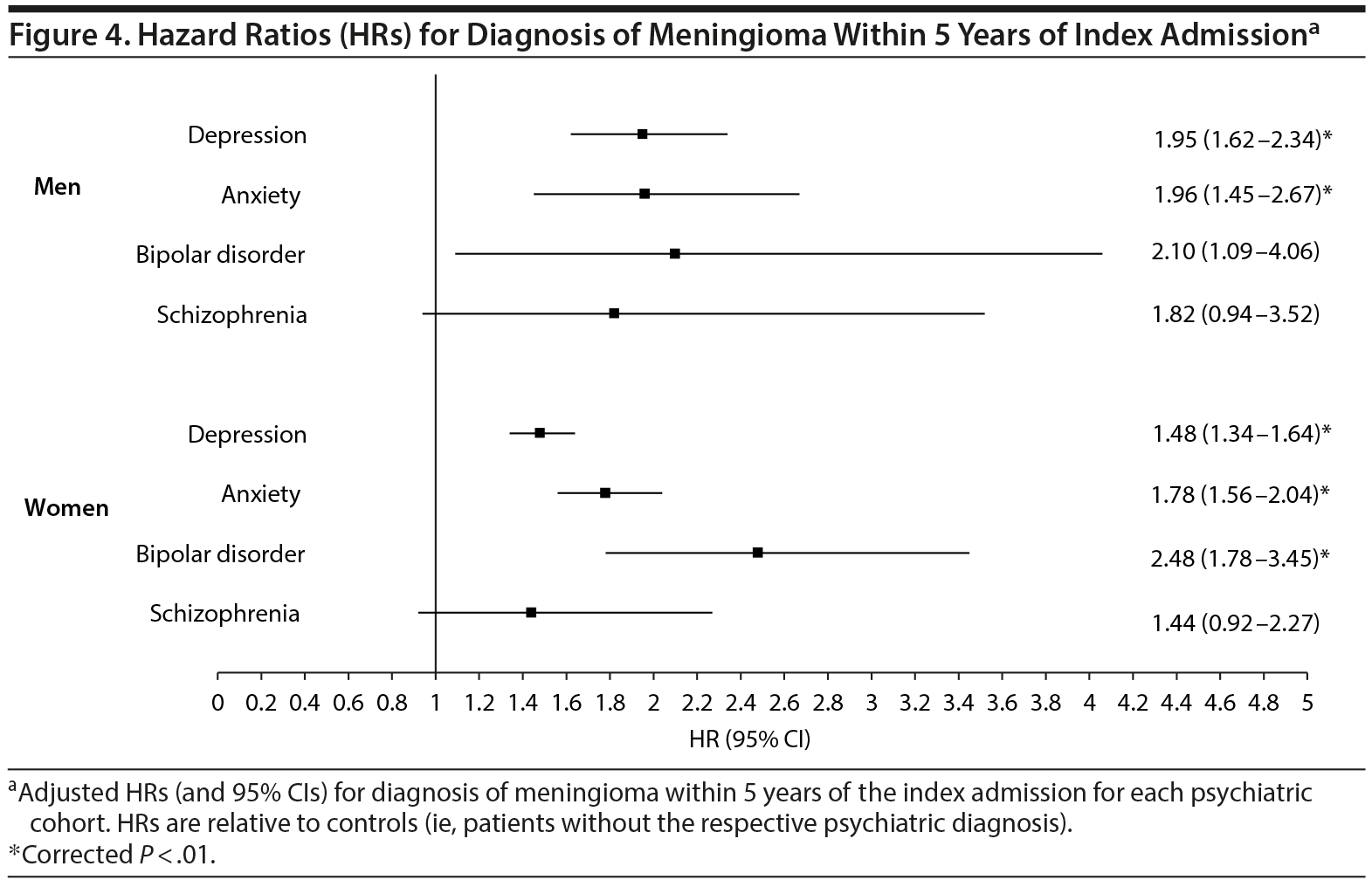
Given the known disparity in meningioma distribution between men and women,15 a gender-stratified analysis was subsequently performed to determine the risk for meningioma diagnosis for all psychiatric cohorts. This analysis revealed an increased risk for meningioma diagnosis among both genders for patients with depression or anxiety; however, meningioma diagnosis risk remained elevated in bipolar patients only among women (P < .01, Figure 4).
DISCUSSION
This study is the first to describe the 5-year prospective incidence of intracranial tumor diagnosis for various psychiatric cohorts in the United States. The study demonstrates that the new documentation of certain psychiatric diagnoses—namely depression, anxiety, or bipolar disorder—during an inpatient admission for any reason is associated with an increased risk of being diagnosed with an intracranial tumor within the next 5 years. More specifically, it demonstrates that certain psychiatric diagnoses are associated with an increased risk of being diagnosed with a subset of intracranial tumors: primary benign neoplasms and meningiomas. This association should broaden the diagnostic differential and heighten awareness for primary care providers when caring for either psychiatric or brain tumor patients.
As mentioned previously, the association between psychiatric diagnosis and intracranial tumor can be considered in 1 of 2 major contexts. The first suggests that certain psychiatric diagnoses may serve as risk factors for subsequent brain tumor development, while the second reverses the causal relationship to consider psychiatric symptoms as harbingers of an underlying proliferating neoplasm.
Our observation that only specific tumor subtypes were more likely among certain psychiatric cohorts sheds light on this evolving debate. Primary benign neoplasms and meningiomas—which we found associated with preexisting depression and anxiety—are tumors that behave fundamentally differently than primary malignant neoplasms of the brain, for which we were unable to demonstrate any association. Most importantly, both benign neoplasms and meningiomas are known to grow at a slower rate than malignant neoplasms, such as glioblastoma, of the brain,16,17 making them less likely to present initially with focal neurologic deficits or signs of elevated intracranial pressure than a faster-growing tumor such as glioblastoma.18 As has been noted in several case reports,11,13,19 particularly for tumors located in the relatively noneloquent frontal lobes, a slow-growing intracranial tumor may not produce any symptoms other than a change in personality until it reaches considerable size. Unfortunately, the OSHPD dataset does not include data that are sufficiently granular to address the issue of precise tumor location. Nevertheless, the disparity in risk between fast- and slow-growing tumors would suggest that these psychiatric diagnoses represent an early manifestation of certain types of slow-growing intracranial tumors. In the setting of an otherwise negative psychiatric workup, this finding may serve as justification for cranial imaging studies, particularly in older female patients.
To attribute this association to differences in tumor growth rate, however, requires addressing our findings pertaining to cranial nerve tumors, which also lack the rapid growth characteristics seen in primary malignant neoplasms such as glioblastoma.20,21 To date, growth patterns of cranial nerve tumors remain poorly understood; however, for a large majority of tumors, slow or even stagnant growth has led to practice guidelines recommending a “wait and watch” approach to their management.22 That our findings failed to show any association with this tumor subtype (see Figure 3) may be a reflection of this stagnant growth unique to cranial nerve tumors, or a predilection for these tumors to present with focal cranial nerve deficits,23 which may preclude observing any change in personality before a diagnosis is made.
Of course, differences in tumor growth rate alone may not definitively establish that psychiatric symptoms are merely an early manifestation of slow-growing tumors. We must also consider the notion that psychiatric illness may predispose to a biological cause in the development of certain tumor types. From an immunologic standpoint, the relative immunosuppression experienced by patients under psychiatric stress24 could render these patients more likely to develop an intracranial tumor. It is known, for instance, that chemically immunosuppressed patients—such as those receiving an organ transplant—are at higher risk for certain de novo neoplasms25,26; however, this risk has not been firmly established for intracranial tumors in particular. This argument, however, is undermined by our findings. One would expect, given this theory of neoplastic proliferation in the immunosuppressed patient, to find an increase in incidence of all tumor types, which we do not see.
From a hormonal standpoint, meningiomas in particular have been theorized to grow in response to endogenous hormones, including circulating glucocorticoids, estrogen, and progesterone.27 In fact, the disparity in meningioma incidence observed between men and women has been ascribed largely to the progrowth effects of estrogen and progesterone.28-30 Given that cortisol levels are thought to be elevated in states of stress or after traumatic events,6,7 a psychiatric illness could arguably accelerate the growth of a preexisting tumor, rendering it large enough to be detected and diagnosed. While we must concede this argument as a potential contributor to our findings, the results of our gender-stratified analysis contend the notion that circulating estrogens specifically play an overwhelming role linking psychiatric disease to intracranial tumor.
Finally, we must consider the question of risk factors shared between psychiatric illness and certain types of brain tumors. X-ray exposure, head trauma,31,32 occupation, and diet all have controversial, but potential, links to meningioma development.29 Given that certain psychiatric illnesses have been shown to associate with an increased risk for traumatic brain injury33 as well as with various lifestyle choices including occupation and diet, our findings may simply reflect a mediator shared among patients with psychiatric illnesses. This argument does not, however, explain our observed finding for both meningioma and primary benign neoplasms. Furthermore, any surveillance bias favoring psychiatric patients (due to more head trauma, for instance) would theoretically increase one’s risk for all tumor types. The fact that we see an increased risk for only certain tumor subtypes, therefore, minimizes the argument of surveillance bias.
This study has several strengths. First, its use of a statewide population-based dataset with large sample size and multiple years of follow-up data allows for substantial power generation when describing the incidence of brain tumors for various psychiatric cohorts. Second, it relies on consistent metrics (ie, ICD-9-CM codes) to determine both risk factor and outcomes status, thereby minimizing variability and subjectivity. Moreover, to the extent that any variability exists in these data, one would anticipate a reduction in finding significance due to an increase in error variance; reliance on a more reliable metric would theoretically bolster our positive findings.
We acknowledge several limitations in this study. First, our conclusions are derived from retrospective data, a factor that necessarily limits our ability to draw causal inferences. Second, its reliance on inpatient admission records restricts our available patient sample; all included patients were necessarily hospitalized at least once during our study period, which we recognize is not reflective of the entire population. Third, our analysis relies on a relatively short follow-up interval of 5 years, which necessarily influences our understanding of risk profiles for tumors of differing growth rates. Finally, we rely upon third-party data to investigate our research question of interest, which prevents us from including important covariates such as symptom severity or medication compliance when considering strength of association or potential mediators of effect. Future research is warranted to further characterize this interface between psychiatric illness and malignancy.
CONCLUSION
Our findings support an association between certain psychiatric diagnoses and subsequent brain tumor diagnosis, which should not go overlooked, particularly in the primary care setting. Moreover, our findings narrow this association to specific tumor subtypes—namely primary benign neoplasm and meningioma. In the context of an ongoing debate over the relationship between brain tumors and preceding psychiatric pathology, we feel that our findings lend credence to the argument for retrograde causality, wherein specifically slow-growing tumors may present with behavioral disturbances before becoming overtly symptomatic.
Submitted: July 31, 2016; accepted October 20, 2016.
Published online: December 15, 2016.
Potential conflicts of interest: None.
Funding/support: The project described was partially supported by National Institutes of Health grant 1TL1TR001443 (awarded to Ms Tringale and Mr Wilson).
Role of the sponsor: The National Institutes of Health held no direct role relating to the oversight or completion of this project.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Previous presentation: The project described was presented as an electronic poster at the American Association of Neurological Surgeons Annual Scientific Meeting; May 30, 2016; Chicago, Illinois.
Additional information: The California Office of Statewide Health Planning and Development (OSHPD) longitudinal inpatient-discharge administrative database is available online (obtained from the State of California OSHPD: http://www.oshpd.ca.gov/HID/Products/PatDischargeData/PublicDataSet/). In California, each time a patient is treated in a licensed acute care hospital, a record is submitted to the OSHPD database.
Supplementary material: See accompanying pages.
REFERENCES
1. Pelletier G, Verhoef MJ, Khatri N, et al. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol. 2002;57(1):41-49. PubMed doi:10.1023/A:1015728825642
2. Arnold SD, Forman LM, Brigidi BD, et al. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro-oncol. 2008;10(2):171-181. PubMed doi:10.1215/15228517-2007-057
3. Bunevicius A, Deltuva V, Tamasauskas S, et al. Screening for psychological distress in neurosurgical brain tumor patients using the Patient Health Questionnaire-2. Psychooncology. 2013;22(8):1895-1900. PubMed doi:10.1002/pon.3237
4. Garssen B, Goodkin K. On the role of immunological factors as mediators between psychosocial factors and cancer progression. Psychiatry Res. 1999;85(1):51-61. PubMed doi:10.1016/S0165-1781(99)00008-6
5. Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617-625. PubMed doi:10.1016/S1470-2045(04)01597-9
6. Sachar EJ, Hellman L, Fukushima DK, et al. Cortisol production in depressive illness: a clinical and biochemical clarification. Arch Gen Psychiatry. 1970;23(4):289-298. PubMed doi:10.1001/archpsyc.1970.01750040001001
7. Strickland PL, Deakin JFW, Percival C, et al. Bio-social origins of depression in the community: interactions between social adversity, cortisol and serotonin neurotransmission. Br J Psychiatry. 2002;180(2):168-173. PubMed doi:10.1192/bjp.180.2.168
8. Autry AE, Monteggia LM. Epigenetics in suicide and depression. Biol Psychiatry. 2009;66(9):812-813. PubMed doi:10.1016/j.biopsych.2009.08.033
9. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349. PubMed doi:10.1586/14737175.7.4.343
10. Ristić DI, Vesna P, Sanja P, et al. Brain tumors in patients primarily treated psychiatrically. Vojnosanit Pregl. 2011;68(9):809-814. PubMed doi:10.2298/VSP1109809I
11. Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285. 10.5498/wjp.v5.i3.273 PubMed
12. Madhusoodanan S, Opler MGA, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? a meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536. PubMed doi:10.1586/ern.10.94
13. Yakhmi S, Sidhu BS, Kaur J, et al. Diagnosis of frontal meningioma presenting with psychiatric symptoms. Indian J Psychiatry. 2015;57(1):91-93. PubMed doi:10.4103/0019-5545.148534
14. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307-314. PubMed doi:10.1007/s11060-010-0386-3 doi: 10.1007/s11060-010-0386-3
15. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307-314. PubMed doi:10.1007/s11060-010-0386-3
16. Hoshino T. A commentary on the biology and growth kinetics of low-grade and high-grade gliomas. J Neurosurg. 1984;61(5):895-900. PubMed doi:10.3171/jns.1984.61.5.0895
17. Go RS, Taylor BV, Kimmel DW. The natural history of asymptomatic meningiomas in Olmsted County, Minnesota. Neurology. 1998;51(6):1718-1720. PubMed doi:10.1212/WNL.51.6.1718
18. Adhiyaman V, Meara J. Meningioma presenting as bilateral parkinsonism. Age Ageing. 2003;32(4):456-458. PubMed doi:10.1093/ageing/32.4.456
19. Maurice-Williams RS, Dunwoody G. Late diagnosis of frontal meningiomas presenting with psychiatric symptoms. Br Med J (Clin Res Ed). 1988;296(6639):1785-1786. PubMed doi:10.1136/bmj.296.6639.1785
20. Nikolopoulos TP, Fortnum H, O’ Donoghue G, et al. Acoustic neuroma growth: a systematic review of the evidence. Otol Neurotol. 2010;31(3):478-485. PubMed doi:10.1097/MAO.0b013e3181d279a3
21. O’ Reilly BF, Mehanna H, Kishore A, et al. Growth rate of non-vestibular intracranial schwannomas. Clin Otolaryngol Allied Sci. 2004;29(1):94-97. PubMed doi:10.1111/j.1365-2273.2004.00770.x
22. Smouha EE, Yoo M, Mohr K, et al. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope. 2005;115(3):450-454. PubMed doi:10.1097/00005537-200503000-00011
23. Selesnick SH, Jackler RK, Pitts LW. The changing clinical presentation of acoustic tumors in the MRI era. Laryngoscope. 1993;103(4 pt 1):431-436. PubMed doi:10.1002/lary.5541030412
24. Kiecolt-Glaser JK, Garner W, Speicher C, et al. Psychosocial modifiers of immunocompetence in medical students. Psychosom Med. 1984;46(1):7-14. PubMed doi:10.1097/00006842-198401000-00003
25. Penn I, Starzl TE. Immunosuppression and cancer. Transplant Proc. 1973;5(1):943-947. PubMed
26. Serraino D, Piselli P, Busnach G, et al; Immunosuppression and Cancer Study Group. Risk of cancer following immunosuppression in organ transplant recipients and in HIV-positive individuals in southern Europe. Eur J Cancer. 2007;43(14):2117-2123. PubMed doi:10.1016/j.ejca.2007.07.015
27. Carroll RS, Zhang J, Dashner K, et al. Progesterone and glucocorticoid receptor activation in meningiomas. Neurosurgery. 1995;37(1):92-97. PubMed doi:10.1227/00006123-199507000-00014
28. Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-oncol. 2012;14(suppl 5):v1-v49. PubMed doi:10.1093/neuonc/nos218
29. Claus EB, Bondy ML, Schildkraut JM, et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57(6):1088-1095, discussion 1088-1095. PubMed doi:10.1227/01.NEU.0000188281.91351.B9
30. Vadivelu S, Sharer L, Schulder M. Regression of multiple intracranial meningiomas after cessation of long-term progesterone agonist therapy. J Neurosurg. 2010;112(5):920-924. PubMed doi:10.3171/2009.8.JNS09201
31. Phillips LE, Koepsell TD, van Belle G, et al. History of head trauma and risk of intracranial meningioma: population-based case-control study. Neurology. 2002;58(12):1849-1852. PubMed doi:10.1212/WNL.58.12.1849
32. Annegers JF, Laws ER Jr, Kurland LT, et al. Head trauma and subsequent brain tumors. Neurosurgery. 1979;4(3):203-206. PubMed doi:10.1227/00006123-197903000-00001
33. Fann JR, Leonetti A, Jaffe K, et al. Psychiatric illness and subsequent traumatic brain injury: a case control study. J Neurol Neurosurg Psychiatry. 2002;72(5):615-620. PubMed doi:10.1136/jnnp.72.5.615
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top