Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Psychogenic polydipsia (PPD), characterized by polydipsia and polyuria, has been commonly identified in patients with psychiatric disorders. It is estimated that up to 20% of psychiatric patients have PPD, with rates being highest among those with schizophrenia. However, cases describing PPD in patients with anorexia nervosa are rare.
Psychogenic Polydipsia in a Woman With Anorexia Nervosa: Case Report and Recommendations
To the Editor: Psychogenic polydipsia (PPD), characterized by polydipsia and polyuria, has been commonly identified in patients with psychiatric disorders. It is estimated that up to 20% of psychiatric patients have PPD, with rates being highest among those with schizophrenia.1 However, cases describing PPD in patients with anorexia nervosa are rare. While many theories concerning the pathophysiology of PPD have been posed, the underlying mechanisms remain to be established.2 Cases of concurrent PPD with both eating disorders and obsessive-compulsive disorder (OCD) may help better elucidate the etiology of PPD. This condition classically causes decreased serum osmolality (< 280 mOsm/kg) and decreased urine osmolality (< 100 mOsm/kg) with varying degrees of hyponatremia, which can have devastating sequelae, including altered mental status, seizures, cerebral edema, coma, and death.3 We present a patient with concurrent PPD and anorexia nervosa admitted for acute symptomatic hyponatremia. We also examine previous case reports in literature, explore the underlying etiology of PPD, and propose a strategy for successful clinical management of PPD in the setting of anorexia nervosa.
A literature search revealed 8 articles related to PPD in patients with anorexia nervosa.4-11 The mean age at presentation was 18 ± 4 (range, 13-26) years. The cases suggest a multifactorial pathogenesis, involving dysfunctional hypothalamic thirst regulation and excessive water intake as an attempt to maintain weight or control anxiety. They also highlight the importance of recognizing hyponatremia as a potentially dangerous complication of compulsive water drinking in patients with anorexia nervosa. However, none of the articles discussed a patient with a history of OCD. The articles were also limited in their discussion of optimal pharmacologic agents for treatment of these patients.
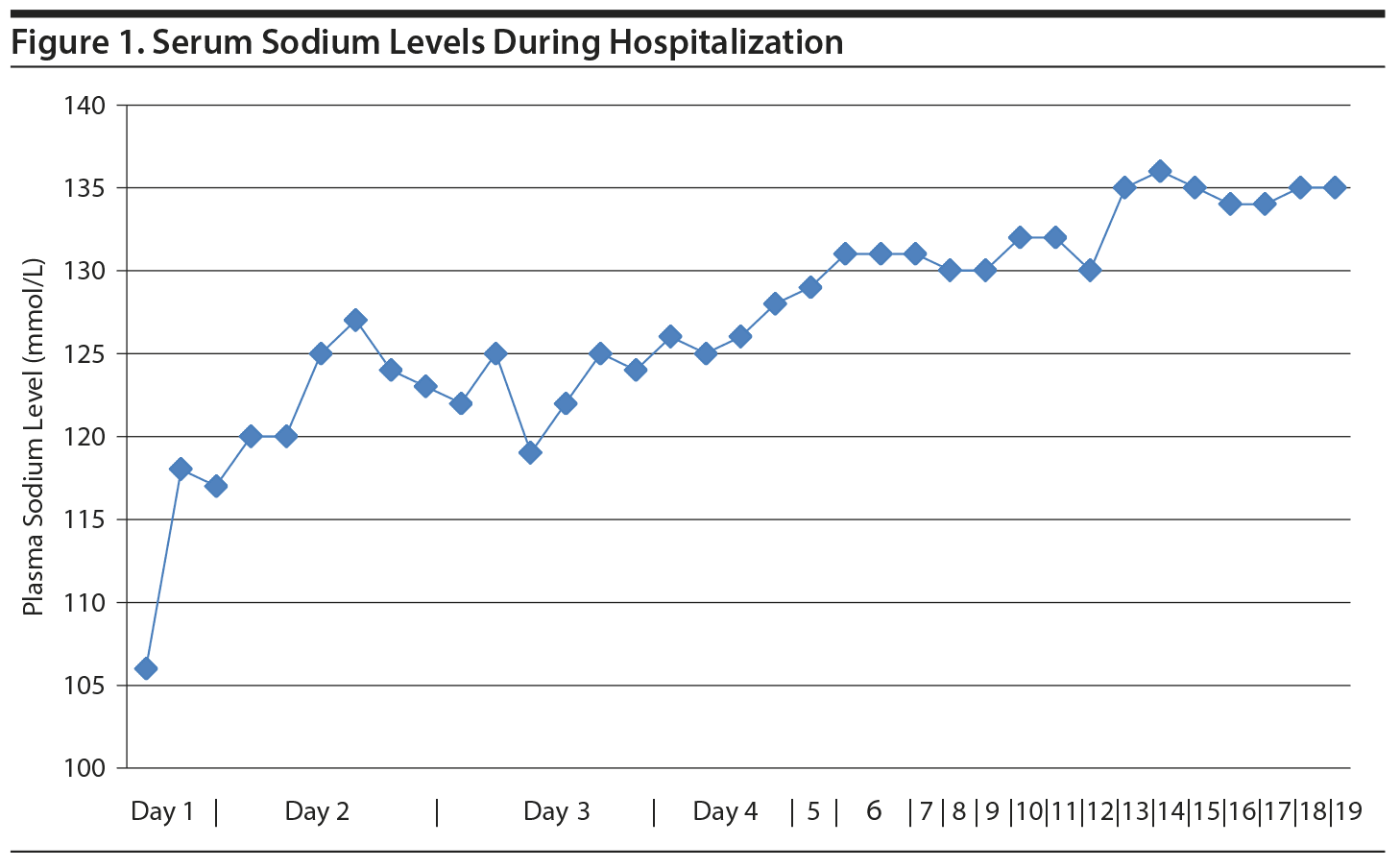
Case report. A 26-year-old woman with a history of anorexia nervosa, PPD, anxiety, depression, and OCD presented to our institution with complaints of light-headedness, abdominal pain, nausea, and several episodes of emesis for 2 days. She also described increased panic attacks and depressed mood for 2 weeks. She was found to have a serum sodium level of 106 mmol/L (normal range, 136-145 mmol/L). The patient was admitted to the medical intensive care unit (MICU), and the psychiatry team was consulted. On admission, the patient endorsed drinking up to 3 gallons (11-12 liters) per day. She stated this excessive drinking was often triggered by psychological stressors that included anxiety about eating at mealtimes and experiencing difficult situations at home and at work. The patient lived with her parents and 10 siblings. She had many responsibilities at home, and endorsed frequent arguments with siblings. She also maintained a vegetarian diet and admitted to eating a select variety of vegetables. The patient was taking no psychiatric medications at the time of admission and had discontinued all medications for 9 months. During the preceding 7 years, she had 8 hospitalizations, including 4 MICU admissions, for hyponatremia secondary to PPD. Previous medication trials included paroxetine and fluoxetine, which she had found helpful for depression and anxiety symptoms, as well as citalopram, clonazepam, quetiapine, temazepam, lorazepam, dronabinol, and gabapentin.
On physical examination, her vitals were within normal limits and body mass index (BMI; measured as kg/m2) was 14.6. A mental status examination was conducted. She appeared older than her stated age, and her hair was thin and brittle, but she was dressed appropriately with fair grooming and hygiene. She was initially dismissive of her condition and guarded, with poor eye contact. Her speech was normal in rate and rhythm, she stated her mood was "a little nervous," and her affect appeared anxious. Her thought process was linear and goal-oriented with tight associations. She exhibited poor insight and judgment into her condition and the behaviors that contributed to her physical symptoms and laboratory abnormalities. She reported anhedonia, guilt, self-criticism, and low energy with trouble sleeping, concentrating, and eating. She also disclosed recent thoughts of nonspecific suicidal ideation.
Other laboratory findings on admission included a potassium of 2.9 mmol/L (normal range, 3.5-5.1 mmol/L), chloride of 72 mmol/L (normal range, 98-107 mmol/L), and magnesium of 1.2 mg/dL (normal range, 1.9-2.7 mg/dL). Her serum osmolality was 245 mOsm/kg (normal range, 278-305 mOsm/kg). Hemoglobin was 10.1 g/dL (normal range, 11.5-15.0 g/dL) with a mean corpuscular volume (MCV) of 75.2 μm3 (normal range, 81.5-97.0 μm3). Results from the toxicology screen were negative, and a urinalysis revealed a urine osmolality of 30 mOsm/kg (normal range, 50-1,200 mOsm/kg). She was diagnosed according to ICD-10 with dilutional hyponatremia (E87.1) due to PPD (R63.1).
In the intensive care unit, she was placed on a fluid restriction of 2 L/day and given intravenous normal saline. Her sodium rapidly increased to 118 mmol/L in the first 6 hours and subsequently to 120 mmol/L in the first 24-hour period. Recognizing the risk of neurologic symptoms with an abrupt rise in sodium, adjustments were made by the medical team to address this concern. Other electrolyte imbalances were repleted according to standard protocols. On hospital day 6, her serum sodium increased to 131 mmol/L, and she was transferred to the psychiatric unit.
The patient was placed on a 72-hour legal hold (5150) and a subsequent 14-day hold (5250) for grave disability. (The 5150 and 5250 are sections of the California Welfare and Institutions Code that allow for involuntary civil commitment of persons with a mental disorder or chronic alcoholism who are a danger to themselves or others.) Fluid restriction was maintained at 1.2 L/day, although the patient frequently attempted to drink more water while brushing teeth and showering. She required a sitter to monitor her behavior. A dietician was also consulted for caloric and nutritional optimization. The patient was counseled on various medications for mood that would also have appetite stimulant and weight gain properties, ie, olanzapine and mirtazapine. She refused these medications due to anxiety about weight gain but was amenable to starting paroxetine. She was started on paroxetine 20 mg, but developed headaches and blurry vision and requested to be placed on fluoxetine 20 mg, which had worked well for her in the past. Additionally, she was encouraged to broaden her dietary choices, and her diet was supplemented with fish oil 3 times daily. She was also given lorazepam prior to each meal to decrease anxiety associated with eating.
While in the psychiatric unit, the patient remained stable and her serum sodium level normalized to 136 mmol/L on hospital day 14 (Figure 1). The patient perseverated on her daily sodium values and displayed obsessive behaviors toward counting her daily fluid intake. At times she became oppositional and bargained with staff for various forms of oral fluids. She also frequently demonstrated psychiatric splitting to various hospital staff. Her food preferences remained particular; however, she regularly met daily calorie and protein goals while in the unit. Her BMI increased to 16.7 at the time of discharge. Her mood steadily improved. She described more control over her impulses to drink water and was able to manage her fluid intake independently.
We present a patient with a history of anorexia nervosa, OCD, anxiety, depression, and previous hospitalizations for PPD, admitted for profound hyponatremia. This case draws attention to the importance of recognizing PPD as a potential cause of electrolyte imbalance in patients with eating disorders. The pathogenesis of PPD is believed to involve thirst dysregulation, in which a lower thirst threshold leads to compulsive drinking, thereby decreasing the tonicity of blood.2 In turn, antidiuretic hormone (ADH) is inhibited, which leads to significant renal excretion of fluids and thus prevents blood from becoming too dilute, perpetuating a cycle of polydipsia and polyuria.12 However, as patients continue to have extreme thirst, they eventually drink beyond the kidneys’ capacity to dilute urine, resulting in hyponatremia. If fluid intake is large and rapid, acute hyponatremia, with sodium levels falling below 125 mmol/L, develops within 24 hours and the patient presents with clinical symptoms of nausea, malaise, headache, lethargy, and obtundation.13
In addition, a review of previously existing reports of patients with anorexia and PPD suggests that, as in this case, the pathology also involves compulsive tendencies with the aim of suppressing hunger and controlling appetite. Patients may drink as an attempt to pacify anxiety associated with their body image, eliminate toxins, and maintain a low target weight.4,11 Water intake and diuresis may also represent a form of binging and purging, respectively, in a subtype of patients with anorexia.4 Interestingly, 1 study14 showed that two-thirds of patients with eating disorders also had at least 1 anxiety disorder, with OCD as the most common, as seen in 41% of these individuals. PPD has previously been proposed to be a variant of OCD in patients with eating disorders.15 Our case supports this notion as she had emotionally distressing ruminations that were relieved by compulsive drinking behaviors. Because anorexia nervosa commonly coexists with depression, anxiety, or OCD, patients may also be taking agents associated with SIADH (syndrome of inappropriate antidiuretic hormone secretion) or anticholinergic side effects like dry mouth, which may further contribute to increased thirst.16
While there is no gold standard for treating PPD, fluid restriction for correction of acute hyponatremia and careful medication selection play a critical role in successful therapy.2 In addition to recognizing PPD as a potentially dangerous comorbid condition, it is important to routinely monitor fluid intake, serum electrolytes, and weight changes in patients with eating disorders. Clinical assessment of hyponatremia in patients with a history of anorexia nervosa should also include urine osmolality and specific gravity measures. Providers should be aware that patients may have manipulative tendencies, often attempting to underreport or cheat on their drinking or urine collection quantities or weight.8 In inpatient settings, one-to-one observation by a patient sitter to monitor for water abuse may be helpful.
Thorough review of antidepressant and antipsychotic medications with special attention to side effect profiles is also important when considering pharmacologic intervention for patients with concurrent psychiatric conditions and PPD, as many commonly prescribed psychopharmacologic agents are associated with hyponatremia. Recently, a retrospective cohort study17 of approximately 70,000 individuals explored the association between various antidepressant classes and hyponatremia. The strongest association was found with selective serotonin reuptake inhibitors (SSRIs), with incidence rate ratios for tricyclic antidepressants and SSRIs at similar levels: citalopram at 7.8; paroxetine, 6.18; fluoxetine, 5.61; nortriptyline, 5.26; clomipramine, 4.93; sertraline, 4.53; escitalopram, 3.74; and amitriptyline, 3.36; with the lowest associations with mirtazapine at 2.95 and among the serotonin-norepinephrine reuptake inhibitors (SNRIs) venlafaxine at 2.90 and duloxetine at 2.05. Our patient refused mirtazapine due to anxiety about weight gain and felt most comfortable taking her previous home medication, fluoxetine; as such, this agent was maintained with the addition of fish oil and lorazepam to successfully decrease mealtime anxiety. Data suggest that highly unsaturated fatty acids modulate brain signaling in dopaminergic and serotonergic pathways through monoamine regulation.18 Because patients with anorexia nervosa and OCD display dysfunctional thalamofrontal circuits and deficiencies in essential fatty acids, fish oil supplementation may be beneficial in this cohort.19
Lastly, although our patient refused olanzapine, many patients with anorexia nervosa are also treated with this agent due to its association with weight gain. One case report20 also showed benefit with olanzapine in a patient with polydipsia. In such patients, it is important to be aware of an increased risk of hospitalization with hyponatremia within 30 days of initiating treatment with an atypical antipsychotic.21
In summary, we report a patient with PPD, anorexia nervosa, and OCD who responded to a multipronged approach of fluid restriction, behavioral monitoring, and anxiety control with pharmacologic agents. This case serves to raise awareness of the co-occurrence of PPD with anorexia nervosa and OCD. As highlighted by our patient’s perplexed thoughts and compulsive drinking habits, this report also suggests the merit of exploring PPD as a variant of OCD. Future studies exploring the relationship between hyponatremia and antidepressant and antipsychotic drugs will be important in clarifying their role in the management of patients with PPD. As further highlighted by this case, careful monitoring, treatment, and follow-up are especially important as patients may have a relapsing course.
References
1. de Leon J. Polydipsia: a study in a long-term psychiatric unit. Eur Arch Psychiatry Clin Neurosci. 2003;253(1):37-39. PubMed CrossRef
2. Dundas B, Harris M, Narasimhan M. Psychogenic polydipsia review: etiology, differential, and treatment. Curr Psychiatry Rep. 2007;9(3):236-241. PubMed CrossRef
3. Sterns RH. Disorders of plasma sodium—causes, consequences, and correction. N Engl J Med. 2015;372(1):55-65. PubMed CrossRef
4. Myers KM, Smith MS. Psychogenic polydipsia in a patient with anorexia nervosa. J Adolesc Health Care. 1985;6(5):404-406. PubMed CrossRef
5. Santonastaso P, Sala A, Favaro A. Water intoxication in anorexia nervosa: a case report. Int J Eat Disord. 1998;24(4):439-442. PubMed CrossRef
6. Gilbert B, Roche JF, Palomera S, et al. Excessive water intake with intoxication as a manifestation of anorexia nervosa. Ann Pediatr (Paris). 1993;40(1):41-43. PubMed
7. Vandepitte M, Vandereycken W. Water intoxication in two girls with anorexia nervosa. Tijdschr Psychiatr. 2008;50(8):545-548. PubMed
8. Jacquin P, Ouvry O, Alvin P. Fatal water intoxication in a young patient with anorexia nervosa. J Adolesc Health. 1992;13(7):631-633. PubMed CrossRef
9. Cuesta MJ, Juan JA, Peralta V. Secondary seizures from water intoxication in anorexia nervosa. Gen Hosp Psychiatry. 1992;14(3):212-213. PubMed CrossRef
10. Silber TJ. Seizures, water intoxication in anorexia nervosa. Psychosomatics. 1984;25(9):705-706. PubMed CrossRef
11. Bahia A, Chu ES, Mehler PS. Polydipsia and hyponatremia in a woman with anorexia nervosa. Int J Eat Disord. 2011;44(2):186-188. PubMed CrossRef
12. Thompson CJ, Edwards C, Baylis P. Osmotic and nonosmotic regulation of thirst and vasopressin secretion in patients with compulsive water drinking. Clin Endocrinol (Oxf). 1991;35(3):221-228. PubMed CrossRef
13. Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307. PubMed
14. Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10(8):573-584. PubMed CrossRef
15. Deas-Nesmith D, Brewerton TD. A case of fluoxetine-responsive psychogenic polydipsia: a variant of obsessive-compulsive disorder? J Nerv Ment Dis. 1992;180(5):338-339. PubMed CrossRef
16. Meynaar IA, Peeters AJ, Mulder AH, et al. Syndrome of inappropriate ADH secretion attributed to the serotonin re- uptake inhibitors, venlafaxine and paroxetine. Neth J Med. 1997;50(6):243-245. PubMed CrossRef
17. Leth-Moller K, Hansen A, Torstensson M, et al. Antidepressants and the risk of hyponatremia: a Danish register-based population study. BMJ Open. 2016;6(5):e011200. PubMed CrossRef
18. Bozzatello P, Brignolo E, De Grandi E, et al. Supplementation with omega-3 fatty acids in psychiatric disorders: a review of literature data. J Clin Med. 2016;5(8):67. PubMed CrossRef
19. Biezonski D, Cha J, Steinglass J, et al. Evidence for thalamocortical circuit abnormalities and associated cognitive dysfunctions in underweight individuals with anorexia nervosa. Neuropsychopharmacology. 2016;41(6):1560-1568. PubMed CrossRef
20. Kruse D, Pantelis C, Rudd R, et al. Treatment of psychogenic polydipsia: comparison of risperidone and olanzapine, and the effects of an adjunctive angiotensin-II receptor blocking drug (irbesartan). Aust N Z J Psychiatry. 2001;35(1):65-68. PubMed CrossRef
21. Gandhi S, McArthur E, Reiss JP, et al. Atypical antipsychotic medications and hyponatremia in older adults: a population-based cohort study. Can J Kidney Health Dis. 2016;3:21. PubMed CrossRef
aDepartment of Psychiatry, UC Irvine Health Neuropsychiatric Center, University of California, Irvine, California
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: The patient provided consent for this case to be published; information has been de-identified to protect anonymity.
Published online: February 8, 2018.
Prim Care Companion CNS Disord 2018;20(1):17l02120
To cite: Torosyan N, Spencer D, Penev S, et al. Psychogenic polydipsia in a woman with anorexia nervosa: case report and recommendations. Prim Care Companion CNS Disord. 2018;20(1):17l02120.
To share: https://doi.org/10.4088/PCC.17l02120
© Copyright 2018 Physicians Postgraduate Press, Inc.
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top