Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Leuprolide acetate is a synthetic analog of naturally occurring gonadotropin-releasing hormone (GnRH) and, when given continuously, inhibits pituitary gonadotropin secretion and suppresses testicular and ovarian steroidogenesis. Leuprolide acetate is indicated for the palliative treatment of advanced prostate cancer and is available as a 1-month, 3-month, 4-month, and 6-month subcutaneous injection. In males, administration of leuprolide acetate results in testosterone levels below castrate threshold (< 50 ng/dL), with this decrease occurring within 2 to 4 weeks after initiation of treatment.' ‹
Psychotic Exacerbation Following Subcutaneous Leuprolide in a Male Patient With Previous History of Schizophrenia
To the Editor: Leuprolide acetate is a synthetic analog of naturally occurring gonadotropin-releasing hormone (GnRH) and, when given continuously, inhibits pituitary gonadotropin secretion and suppresses testicular and ovarian steroidogenesis.1 Leuprolide acetate is indicated for the palliative treatment of advanced prostate cancer and is available as a 1-month, 3-month, 4-month, and 6-month subcutaneous injection. In males, administration of leuprolide acetate results in testosterone levels below castrate threshold (< 50 ng/dL), with this decrease occurring within 2 to 4 weeks after initiation of treatment.1,2
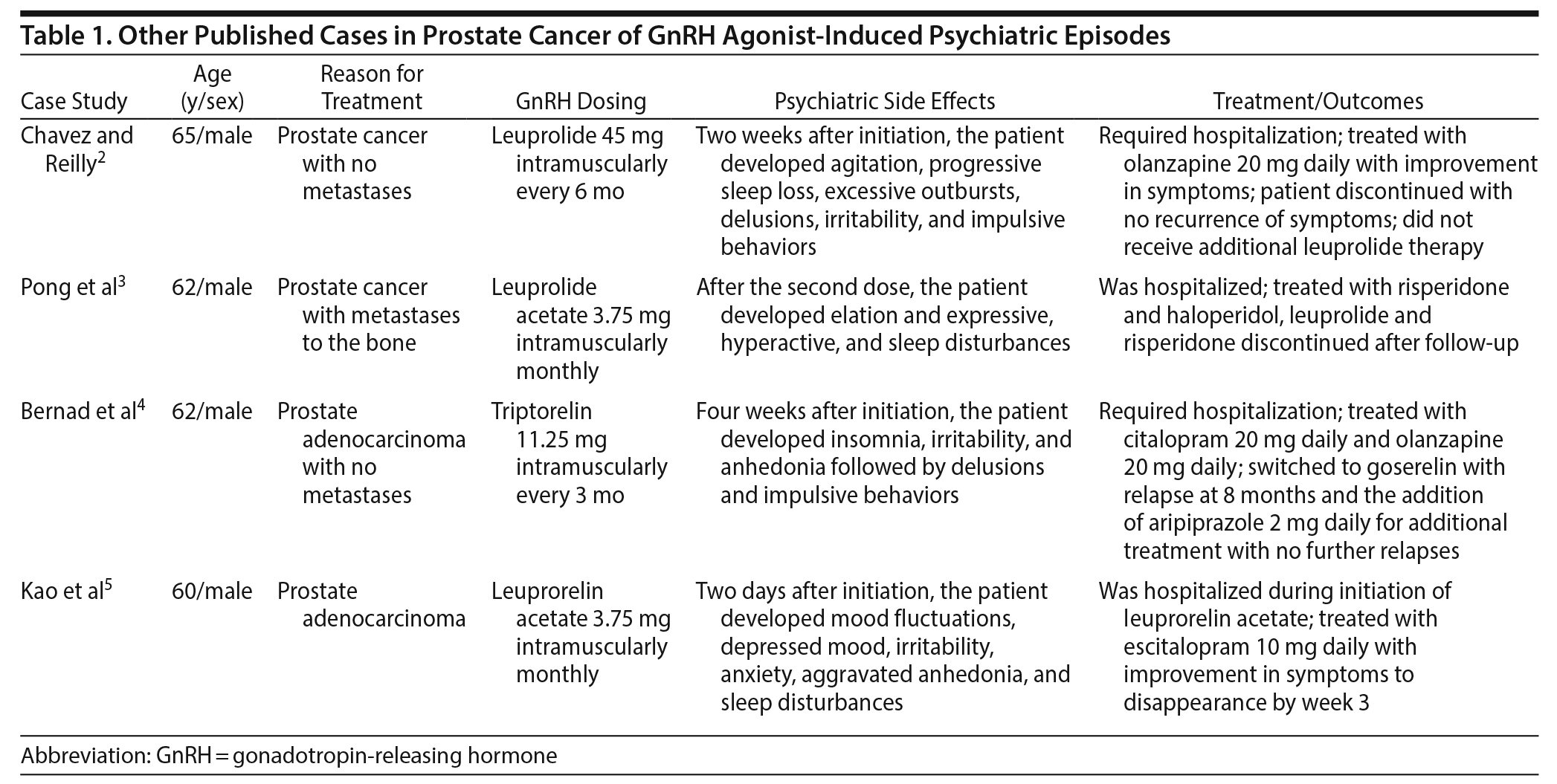
Although there have been reports2-4 in the literature demonstrating patients experiencing emotional lability, depression, mania, and psychotic symptoms with GnRH agonist use, these reports have mainly been in patients with no prior history of psychiatric illness (Table 1). There is only 1 case5 that we identified in the literature of a patient with a previous psychiatric illness who received leuprolide acetate and experienced worsening psychiatric symptoms (Table 1). The leuprolide acetate subcutaneous injection package insert1 lists insomnia and anxiety as occurring in < 2% of patients. Here, we present the case of a man with prior psychiatric history of paranoid schizophrenia who experienced an acute exacerbation of his psychotic symptoms after receiving a 6-month subcutaneous injection of leuprolide acetate.
Case report. A 67-year-old black man presented to the emergency department in March 2016 because of continued acute psychiatric decompensation with homicidal ideations toward his immediate relatives. The patient was discharged from the inpatient psychiatry unit 2 days prior with a similar presentation. The patient exhibited increased irritability, screaming, and frequent episodes of paranoia. Relevant past medical history included a 20-year history of paranoid schizophrenia (DSM-IV criteria), prostate cancer, type 2 diabetes mellitus, and coronary artery disease. The patient was diagnosed with prostate cancer with no metastases in July 2012, and he was able to complete 6 treatments of radiation therapy that ended in February 2013. Due to his continued rise in prostate-specific antigen levels postradiation, leuprolide acetate 45 mg subcutaneously every 6 months was initiated in February 2016.
Presently, all relevant laboratory data were within normal limits. The patient reported no drug allergies, smoked a half pack of cigarettes per day with limited alcohol consumption, and denied current illicit drug use. His current psychotropic medication regimen included haloperidol 15 mg by mouth twice daily and benztropine 1 mg twice a day, which the patient had been stable on for 3 years. The attending psychiatrist decided to increase the patient’s haloperidol dose to 20 mg twice daily. Despite the dose increase of the haloperidol, the patient continued to be irritable, paranoid, and uncooperative with medical staff. Olanzapine 2.5 mg was eventually initiated and titrated to 5 mg daily. With the addition of olanzapine, the patient reported improved sleep and mood and a decrease in paranoia. At discharge, the patient was displaying no paranoia or aggression, denied homicidal ideation, and displayed fair insight into his disease management.
To our knowledge, this is the first case report describing a patient with a previous psychotic illness who received leuprolide therapy and subsequently experienced worsening psychosis after being psychiatrically stable for an extended period of time. Previous research has shown that a decrease in testosterone levels can alter the dopamine system in the prefrontal cortex, which is one of the mechanisms proposed to be associated with psychotic behavior.4,6-8 With this knowledge, it is imperative for health care providers to screen patients for preexisting psychiatric illnesses before initiating GnRH agonist therapy. Screening patients for preexisting mental illness prior to treatment will allow clinicians to choose a shorter-acting formulation to prevent persistent psychiatric exacerbations from occurring throughout therapy while also limiting psychotropic polypharmacy.
References
1. Eligard [package insert]. Fort Collins, CO: Tolmar Pharmaceuticals; 2016.
2. Chavez B, Reilly T. Manic and psychotic symptoms following subcutaneous leuprolide in a male patient with no prior psychiatric history. J Clin Psychiatry. 2010;71(12):1696-1698. PubMed doi:10.4088/JCP.10l06190yel
3. Pong Y, Lu Y, Tsai VF, et al. Acute manic and psychotic symptoms following subcutaneous leuprolide acetate in a male patient without prior psychiatric history: a case report and literature review. Urological Science. 2014;25(1):22-24. doi:10.1016/j.urols.2013.05.010
4. Bernad DM, Dal Pra A, Baule C, et al. New-onset psychosis following androgen deprivation therapy for prostate cancer. Can J Urol. 2013;20(4):6868-6870. PubMed
5. Kao LC, Chen LF, Hsu YC, et al. Escitalopram in treatment of antiandrogen-related mood disturbance in a patient with chronic schizophrenia and adenocarcinoma of the prostate. Gen Hosp Psychiatry. 2015;37(4):e11-e12. PubMed doi:10.1016/j.genhosppsych.2015.03.023
6. Kritzer MF. Effects of acute and chronic gonadectomy on the catecholamine innervation of the cerebral cortex in adult male rats: insensitivity of axons immunoreactive for dopamine-beta-hydroxylase to gonadal steroids, and differential sensitivity of axons immunoreactive for tyrosine hydroxylase to ovarian and testicular hormones. J Comp Neurol. 2000;427(4):617-633. PubMed doi:10.1002/1096-9861(20001127)427:4<617::AID-CNE9>3.0.CO;2-#
7. Kritzer MF. Long-term gonadectomy affects the density of tyrosine hydroxylase- but not dopamine-beta-hydroxylase-, choline acetyltransferase- or serotonin-immunoreactive axons in the medial prefrontal cortices of adult male rats. Cereb Cortex. 2003;13(3):282-296. PubMed doi:10.1093/cercor/13.3.282
8. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549-562. PubMed doi:10.1093/schbul/sbp006
aDepartment of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, Texas
bDepartment of Pharmacy, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas
cDepartment of Psychiatry, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas
Potential conflicts of interest: None.
Funding/support: None.
Published online: April 6, 2017.
Patient consent: Permission was received from the patient to publish this case report.
Prim Care Companion CNS Disord 2017;19(2):16l02038
https://doi.org/10.4088/PCC.16l02038
© Copyright 2017 Physicians Postgraduate Press, Inc.
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top