Objective: To address a gap in the literature for concise recommendations on psychotropic medication monitoring geared to prescribers in primary care psychiatry.
Data Sources: Large institutional guidelines from the United States, United Kingdom, Canada, and Australia/New Zealand combined with manual searches for psychiatric medication monitoring consensus and other recommendations up to January 31, 2018.
Study Selection: Any available guidelines and consensus statements making psychotropic medication monitoring recommendations for treatment of adults and published in English.
Data Extraction: Manual identification of all specific recommendations on psychotropic medication monitoring from the sources.
Results: Psychotropic medication monitoring recommendations vary by source, but there is considerable agreement among English-language sources, which can be readily summarized for teaching and everyday use.
Conclusions: For prescribers working in many disciplines, medication monitoring may be improved by having more ready access to recommendations.

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
‘ ¢ Use monitoring recommendations to provide safe psychotropic prescribing
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in February 2019 and is eligible for AMA PRA Category 1 Creditâ„¢ through February 28, 2021. The latest review of this material was February 2019.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for Alkermes, Harmony Biosciences, Merck, Shire, Supernus, and Sunovion. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
ABSTRACT
Objective: To address a gap in the literature for concise recommendations on psychotropic medication monitoring geared to prescribers in primary care psychiatry.
Data Sources: Large institutional guidelines from the United States, United Kingdom, Canada, and Australia/New Zealand combined with manual searches for psychiatric medication monitoring consensus and other recommendations up to January 31, 2018.
Study Selection: Any available guidelines and consensus statements making psychotropic medication monitoring recommendations for treatment of adults and published in English.
Data Extraction: Manual identification of all specific recommendations on psychotropic medication monitoring from the sources.
Results: Psychotropic medication monitoring recommendations vary by source, but there is considerable agreement among English-language sources, which can be readily summarized for teaching and everyday use.
Conclusions: For prescribers working in many disciplines, medication monitoring may be improved by having more ready access to recommendations.
Prim Care Companion CNS Disord 2019;21(1):18r02324
To cite: Schreiber MA, Armstrong SC, Markman JD. Psychotropic medication monitoring: a review. Prim Care Companion CNS Disord. 2019;21(1):18r02324.
To share: https://doi.org/10.4088/PCC.18r02324
© Copyright 2019 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Behavioral Sciences, University of Washington, Seattle, Washington
*Corresponding author: Matthew A. Schreiber, MD, PhD, Department of Psychiatry and Behavioral Sciences, University of Washington, 1660 S. Columbian Way, S-116-MHC, Seattle, Washington ([email protected]).
In addition to therapeutic benefits, psychotropic medications carry iatrogenic risks. Medication monitoring helps minimize this risk and is an essential part of safe prescribing reflected in professional guidelines and recommendations. These guidelines have never, to our knowledge, been summarized in one place for clinicians’ easy access. We review a wide range of these sources and present monitoring recommendations along with a 1-page quick guide for reference.
As more practitioners prescribe psychotropic medications, it is important to ensure that providers are familiar with medication monitoring recommendations, especially since there is evidence that performance could be improved. For example, 1 study noted that “up to 70% of people taking antipsychotics remain unscreened and untreated”1(p1083) for cardiovascular health issues. In another study,2 patients treated with second-generation antipsychotics in a commercially insured population were monitored at a rate of 38% for glucose and 23% for lipids. While monitoring rates differ across study populations, they are consistently lower than optimal based on recommendations.3-5
The reasons for lower-than-ideal adherence to monitoring guidelines are not well studied.6 A convenient source for this information could provide a useful tool for education about monitoring and serve as a reference for active prescribers in diverse clinical settings—from primary care to specialty mental health clinics—with the aim of improving prescribing performance and, ultimately, patient safety.7
METHODS
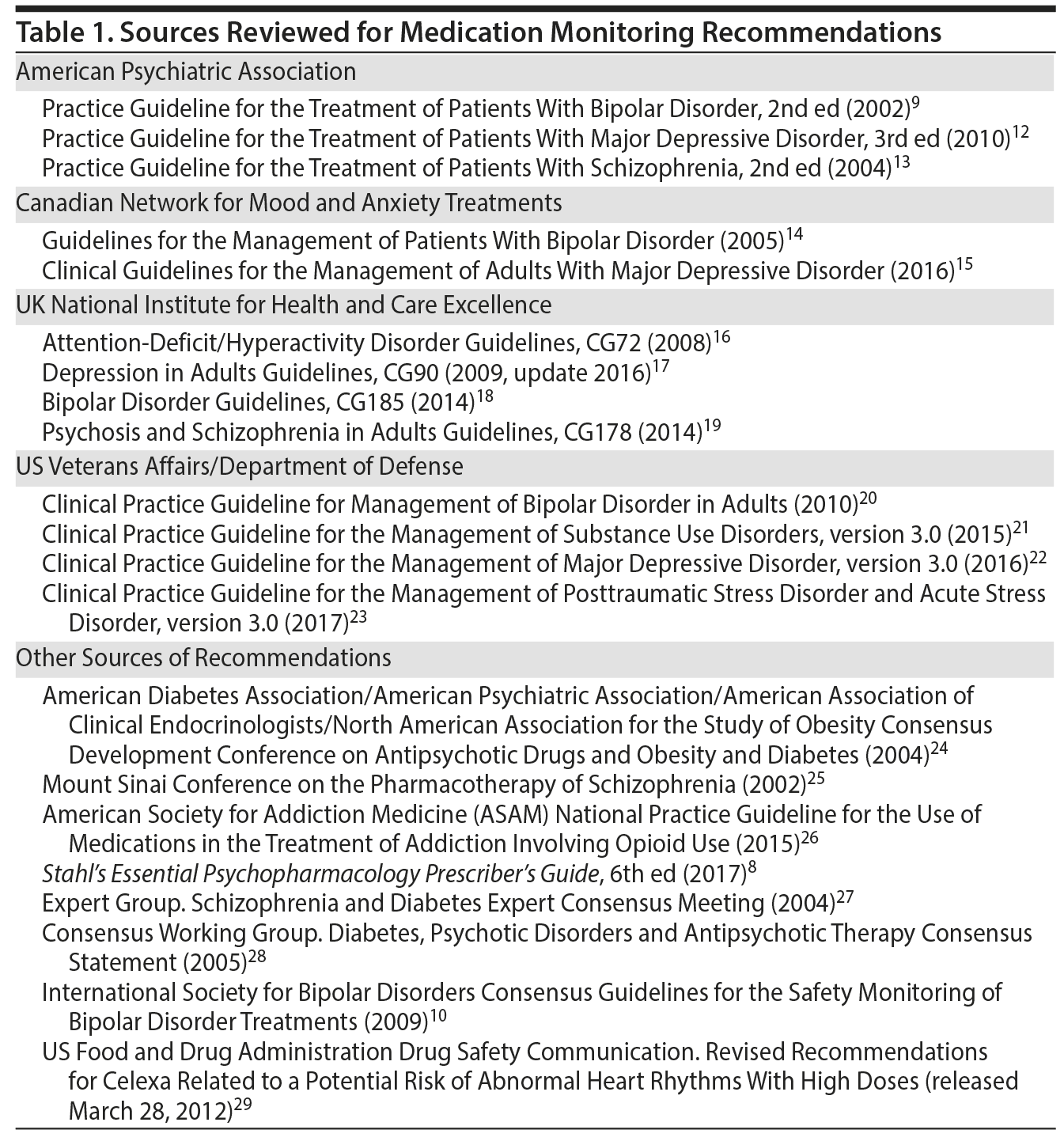
To identify medication monitoring recommendations, major professional organization websites were serached for English-language guidelines relating to major depressive disorder, bipolar disorder, and schizophrenia. Organizations included the American Psychiatric Association (APA), UK National Institute for Health and Care Excellence (NICE), US Veterans Affairs/Department of Defense (VA/DoD), and Canadian Network for Mood and Anxiety Treatments (CANMAT). When guidelines for other conditions contained medication monitoring recommendations, these were also included. To supplement this material, we conducted PubMed and internet searches for terms relating to specific psychiatric conditions paired with terms for guidelines and treatment recommendations. Searches were from inception up to January 31, 2018. Finally, we also incorporated recommendations from Stahl’s Essential Psychopharmacology Prescriber’s Guide,8 acknowledging its widespread use among prescribers and trainees. Within each source, we searched specifically for monitoring recommendations and collected these together.
There is no large evidence base for the effectiveness of specific recommendations, and for this reason, it is not readily possible to apply PRISMA standards to review of this material. Thus, we took a practical, narrative approach of assembling a monitoring quick guide by reviewing the collected guidelines, extracting the recommendations from each guideline, and assessing which recommendations were most frequently made across guidelines. These recommendations appear in bold type in the quick guide. Less frequently made recommendations (eg, did not appear in multiple guidelines but we considered useful for prescribers to consider on the basis of clinical judgment) follow these bolded consensus recommendations in the quick guide and are not in bold type. We also added a few clinically relevant monitoring situations that are not addressed by the reviewed guidelines directly but can be found elsewhere in the clinical literature, for example, monitoring sodium when using some antidepressants. As more research becomes available, it may be possible to specifically determine which recommendations provide the strongest benefit to patients and to consider practical and economic aspects of testing. As this happens, or as guidelines change over time, it would be straightforward to update this quick guide.
Clinical Points
- Better access to monitoring recommendations will improve safe psychotropic prescribing.
- A quick guide to psychotropic monitoring will facilitate teaching and make it easier for providers to remember.
Finally, there are some notable exceptions to what we have included. We do not systematically address use of drug levels for therapeutic, as opposed to medication safety, purposes. We did not include clozapine, which requires participation in a risk evaluation and mitigation strategy program due to its highly specific monitoring requirements. We do not address the merits of these recommendations or provide research material on their utility. Also, it is important to note that the guidelines we reviewed, with few if any exceptions, do not give recommendations on what actions to take with the results. These decisions are made according to the individual clinical judgment of the prescriber ordering the tests.
Definitions of Terms Used by Guidelines
Guidelines often use common shorthand terms for types of laboratory test by category, and this is reflected in the quick guide. Three in particular can be highlighted.
Liver/hepatic function. Guidelines often reference LFTs (liver function tests), which are seldom defined in detail (eg, see APA Work Group on Bipolar Disorder9 and Ng et al10). Consolidating across guidelines, it seems reasonable to suggest that LFTs should at a minimum include aspartate aminotransferase/serum glutamic-oxaloacetic transaminase, alanine transaminase/serum glutamic-pyruvic transaminase, and total bilirubin. LFTs also might include liver synthetic function tests such as international normalized ratio (INR) or albumin, but this is not made clear across available recommendations.
Hematology. Similarly, when recommending a complete blood count, although not consistently explicitly mentioned, we would suggest always obtaining a differential as part of monitoring, in particular due to several medications that have the potential to cause neutropenia or thrombocytopenia.
Thyroid. Thyroid tests are frequently recommended but seldom precisely defined. Most commonly, thyroid-stimulating hormone is the specific test cited, and other tests to further refine assessment of thyroid function are not often discussed but might include free T4 and free T3, as suggested when using thyroid augmentation of antidepressants.11 In general, we have used the most common terminology utilized across sources and leave the specific tests to the judgment of individual clinicians, which may be influenced, for example, by local customary procedures. For this reason, we use the most general terms when referring to these groups of tests.
RESULTS
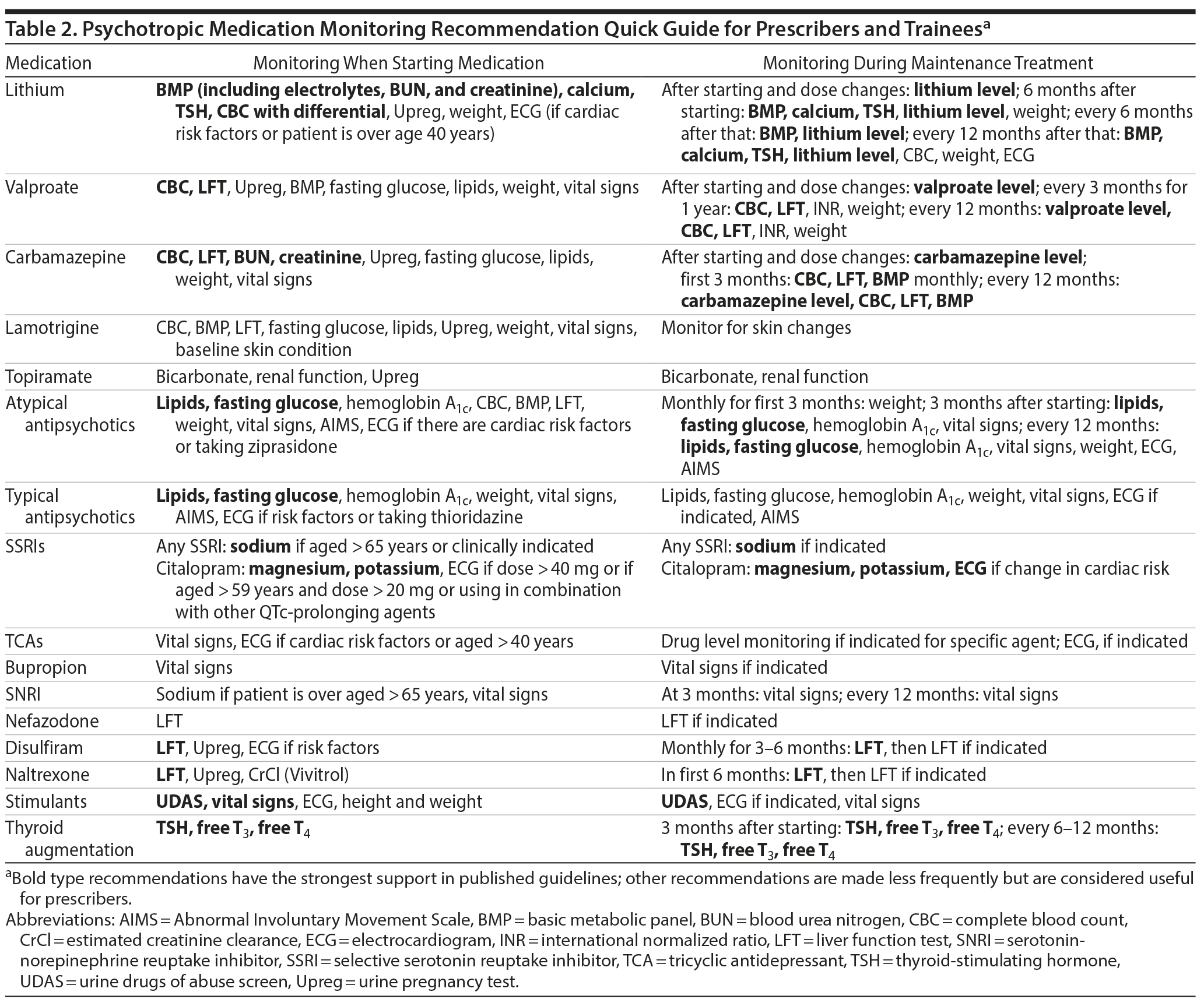
The main guidelines reviewed are listed in Table 1. Overall, there is much agreement across a wide range of sources for the major classes of psychotropics, providing prescribers with a solid basis for medication monitoring recommendations. In Supplementary Table 1, the raw information from all sources reviewed is presented to facilitate comparison. In Table 2, we provide a quick guide based on recommendations that are most frequently found in the source material by medication category. Specific recommendations for individual agents are also shown. In some areas, there may be local prevailing clinical practices that may differ from these recommendations. Also, we review the basic findings for 3 major classes of psychotropics: antipsychotics, mood stabilizers/lithium, and antidepressants.
Antipsychotics
Medication monitoring recommendations for antipsychotics have been highly influenced by specific medication safety recommendations from the 2004 American Diabetes Association (ADA)/APA joint publication of recommended monitoring related to second-generation antipsychotics (SGAs),24 of which clozapine, olanzapine, risperidone, ziprasidone, and aripiprazole were available for consideration at that time. A forerunner to these recommendations from the Mount Sinai Conference on the Pharmacotherapy of Schizophrenia in 2002 offered similar guidance.25 These recommendations focus on the metabolic side effects of SGAs on blood sugar and lipids. Most guidelines recommend that fasting lipid profiles and fasting glucose level be measured at baseline, at 3 months after initiation, at 12 months, and annually thereafter. Because fasting blood glucose is a more direct indicator of glucose metabolism and is used in diabetes diagnosis, this is the recommended measure in most guidelines (including the ADA/APA consensus24).30 In addition, the glucose level will be a more accurate reporter very early in the course of taking an SGA, since hemoglobin A1c levels reflect glucose levels over a period of time.31 NICE guidelines on schizophrenia (2014) recommend using the hemoglobin A1c level to monitor metabolic effects.19 In practice, many clinicians most likely use the hemoglobin A1c level to monitor metabolic effects of atypical antipsychotics, and we include hemoglobin A1c in the summary table to reflect this. However, clinicians should be aware of this discrepancy and try to ensure that fasting glucose and hemoglobin A1c levels are obtained, especially early in the course of treatment. Tracking of weight and vital signs on the same schedule is also recommended. Less consistently, waist circumference is also recommended for monitoring in many guidelines. Of note, NICE guidelines on schizophrenia recommend similar monitoring when treating patients with typical (first-generation) antipsychotics.19
Another major monitoring consideration for antipsychotics is their effect on cardiac conduction, for which there are no well-established guidelines.32 There are many resources for information on QT-prolonging medications (eg, CredibleMeds at www.crediblemeds.org). In practice, clinical concern is focused on medications that are known to have relatively large QT-prolonging potential, particularly among antipsychotics, thioridazine, and ziprasidone; with these medications, more frequent electrocardiogram (ECG) monitoring is recommended.25 More generally, clinical recommendations focus on taking into account patient-specific risk factors including cardiac history, as well as assessment of overall patient regimens for multiple medications with QT-prolonging potential (eg, nonpsychotropics including methadone, quinolones, and others). Similar to pregnancy testing, much is left to the judgment of the individual clinician with regard to which patients should obtain an ECG and when.
Finally, the potential for antipsychotics to cause involuntary movement disorders warrants tracking of patients with the Abnormal Involuntary Movement Scale33), although this is not directly addressed in most guidelines.
Mood Stabilizers and Lithium
Because levels of mood stabilizers and lithium are used to guide treatment (and due to the low therapeutic index of lithium), laboratory monitoring with these agents is well known to psychiatrically trained prescribers but may be less familiar to those who may come into contact with patients in other clinical areas such as primary care. Recommendations for monitoring during lithium use include baseline calcium level due to risk of hyperparathyroidism, creatinine for renal function, and thyroid profiles, with follow-up generally recommended every 6 months. However, there are some differences in approach across guidelines. APA guidelines specify that lithium levels should be monitored 5 to 7 days after dose changes, with no further recommendations on routine monitoring,9 while the VA/DoD recommends that lithium levels be obtained every 6 months.20 NICE advises to check levels every 6 months, increasing surveillance to every 3 months when patients are older or have medical complications raising their risk of adverse lithium effects, if there is poor clinical symptom control, or if previous lithium levels have been greater than 0.8 mEq/L.18 Of note, the International Society for Bipolar Disorders (ISBD)10 has issued detailed treatment guidelines independently. These guidelines10 recommend that lithium levels (along with electrolytes, urea/blood urea nitrogen, and creatinine) be obtained every 3 to 6 months and also provide a wealth of other information on lithium monitoring and use.
For divalproex, recommendations emphasize tracking hepatic adverse effects with liver function tests (although the precise tests are not specified in most guidelines) and hematologic toxicity with complete blood counts at initiation and then every 6 or 12 months. Guidelines do not suggest monitoring liver synthetic function (eg, using the INR), although some clinicians may do this. There are no clear consensus recommendations on metabolic monitoring for divalproex, and guidelines vary from scheduled measurement to only testing when clinically indicated (although this is not specifically defined). For example, CANMAT,14 ISBD,10 and NICE18 recommend tracking weight (and BMI and waist circumference). In turn, clinicians may want to follow other metabolic parameters such as lipids depending on weight changes.
For carbamazepine, recommendations are similar to divalproex overall, as known toxicities for these 2 antiepileptic drugs overlap considerably. Pregnancy risk is discussed here in general terms but is a well-known concern with lithium, divalproex, and carbamazepine in particular among psychotropics.
Antidepressants
There are far fewer recommendations for monitoring with antidepressants; recently published consensus recommendations addressing this are useful.30 The cardiac rhythm effects of antidepressants, in particular tricyclic antidepressants (TCAs) as a class, and citalopram warrant mention, although there is little specific guidance from published guidelines. In particular, there are no consensus guidelines for when to obtain ECGs to monitor these effects. For example, the APA recommends an ECG for patients “with significant cardiac risk factors and patients older than age 50 years” at baseline,12(p40) and more specific recommendations for monitoring after initiating tricyclics in the same guidelines suggest considering a follow-up ECG. Other guidelines do not offer explicit guidance on ECG monitoring with TCAs (outside of overdose settings). In this absence, independent clinical judgment can be used. It seems reasonable to suggest that when prescribing TCAs, ECGs are useful in patients over 50 years of age with known cardiac risk factors, including known cardiac disease or history of abnormal rhythm or with family history of arrhythmia or sudden death (presumed to be due to unspecified cardiac issues, especially congenital long QT syndrome).
In addition to TCAs for which cardiac rhythm effects, especially in overdose, are well known, QT prolongation with citalopram has been a focus of concern, and monitoring recommendations related to this have posed a more complex issue. In a Drug Safety Communication, the US Food and Drug Administration (FDA) in 201229 revised recommendations to suggest “more frequent” ECG monitoring in patients for whom citalopram is not recommended but is considered essential, as well as baseline serum potassium and magnesium measurement in “patients at risk for significant electrolyte disturbances.” In addition, the FDA recommends that citalopram should be discontinued in patients with persistent QTc measurements greater than 500 msec. The FDA does not specify exactly when monitoring ECGs should be done, and this decision is left to clinical judgment.
Other considerations are hyponatremia and weight gain. Particularly with selective serotonin reuptake inhibitor and serotonin-norepinephrine reuptake inhibitor antidepressants, hyponatremia is a risk. Recently, a consensus paper on antidepressant use recommended checking sodium levels (along with electrolytes in general) prior to initiating antidepressants, with repeat testing 3-5 weeks into treatment.30 The same source30 points out that for weight gain, mirtazapine and TCAs pose the most concern and suggests that TCAs be avoided in patients with obesity due to baseline cardiovascular risk.
Medication monitoring for pregnancy. Pregnancy testing is important to single out as a consideration for monitoring. There are 2 distinct considerations: monitoring for pregnancy in patients who may become pregnant and monitoring of medications during pregnancy (once collaborative medication use decisions have been made). There are few consistent guidelines of when to test for pregnancy other than to consider testing (and to document this) in all women of childbearing potential. Some guidelines recommend urine pregnancy tests in some specific situations (eg, when starting lithium). There is no specific guidance on when a serum pregnancy test would be indicated instead due to its higher sensitivity. For monitoring medications in patients who are known to be pregnant, there is little direct guidance on how monitoring should change. However, considering physiologic changes in pregnancy, in general, clinicians should consider closer monitoring of medications with crucial blood levels, particularly lithium, which is very sensitive to volume status.
Other monitoring issues. To help focus the results and make them of the most general use, we have compiled recommendations for commonly used agents and classes of agents, but the absence of a medication does not imply that monitoring is not recommended or required. For example, nefazodone, which is now very infrequently prescribed, requires monitoring for liver inflammation. While no available guidelines address these issues in detail, this information is readily accessible in the literature for specific medications. In addition, in some countries, certain monitoring is required (eg, liver monitoring when using agomelatine), and we do not address this here due to variation across locations.30 Finally, while guidelines make recommendations about monitoring, they do not often address follow-up actions for abnormal laboratory values. In this vein, NICE bipolar disorder guidelines18 state that the ordering professional should ensure further investigations and treatment are offered to the patient but do not offer more specific guidance. A future review on this subject might also be useful for prescribers.
DISCUSSION
By gathering recommendations from a wide variety of sources, we hope to increase their accessibility for both trainees and independent prescribers. While there are some differences among expert recommendations listed in Table 1, which can be reviewed in detail in Supplementary Table 1, there is substantial agreement across sources that we summarize in the quick guide (see Table 2). Monitoring has not typically been a focus for education, but teaching from these summarized recommendations could facilitate better practice of medication reconciliation and provider education to patients, both of which have been the focus in improving medication safety and reducing medication errors.34 In turn, it may be possible to continue to improve performance with regard to safe and effective prescribing for providers across a wide range of fields.
Submitted: May 6, 2018; accepted October 9, 2018.
Published online: February 21, 2019.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Financial disclosure: Drs Schreiber, Armstrong, and Markman have no personal affiliations or financial relationships with any commercial interest to disclose relative to this article.
Funding/support: None.
Disclaimer: The expressed views do not necessarily represent those of the US Department of Veterans Affairs.
Supplementary material: See accompanying pages.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Mangurian C, Newcomer JW, Modlin C, et al. Diabetes and cardiovascular care among people with severe mental illness: a literature review. J Gen Intern Med. 2016;31(9):1083-1091. PubMed CrossRef
2. Morrato EH, Newcomer JW, Kamat S, et al. Metabolic screening after the American Diabetes Association’s consensus statement on antipsychotic drugs and diabetes. Diabetes Care. 2009;32(6):1037-1042. PubMed CrossRef
3. Moeller KE, Rigler SK, Mayorga A, et al. Quality of monitoring for metabolic effects associated with second generation antipsychotics in patients with schizophrenia on public insurance. Schizophr Res. 2011;126(1-3):117-123. PubMed CrossRef
4. Barnett M, VonMuenster S, Wehring H, et al. Assessment of monitoring for glucose and lipid dysregulation in adult Medi-Cal patients newly started on antipsychotics. Ann Clin Psychiatry. 2010;22(1):9-18.
5. Keller WR, Fischer BA, McMahon R, et al. Community adherence to schizophrenia treatment and safety monitoring guidelines. J Nerv Ment Dis. 2014;202(1):6-12. PubMed CrossRef
6. McLaren JL, Brunette MF, McHugo GJ, et al. Monitoring of patients on second-generation antipsychotics: a national survey of child psychiatrists. Psychiatr Serv. 2017;68(9):958-961. PubMed CrossRef
7. Scheiber SC, Kramer TAM, Adamowski SE. Core Competencies for Psychiatric Practice. A Report of the American Board of Psychiatry and Neurology, Inc. Washington, DC: American Psychiatric Association; 2003.
8. Stahl SM. Stahl’s Essential Psychopharmacology Prescriber’s Guide. 6th ed. New York, NY: Cambridge University Pres; 2017.
9. American Psychiatric Association Work Group on Bipolar Disorder. Practice Guideline for the Treatment of Patients With Bipolar Disorder, second edition. 2002. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf.
10. Ng F, Mammen OK, Wilting I, et al; International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) Consensus Guidelines for the Safety Monitoring of Bipolar Disorder Treatments. Bipolar Disord. 2009;11(6):559-595. PubMed CrossRef
11. Rosenthal LJ, Goldner WS, O’ Reardon JP. T3 augmentation in major depressive disorder: safety considerations. Am J Psychiatry. 2011;168(10):1035-1040. PubMed CrossRef
12. American Psychiatric Association Work Group on Major Depressive Disorder. Practice Guideline for the Treatment of Patients With Major Depressive Disorder, third edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. 2010.
13. American Psychiatric Association. Practice Guideline for the Treatment of Patients with Schizophrenia, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/
practice_guidelines/guidelines/schizophrenia.pdf. 2004.
14. Yatham LN, Kennedy SH, O’ Donovan C, et al; Canadian Network for Mood and Anxiety Treatments. Canadian Network for Mood and Anxiety Treatments (CANMAT) Guidelines for the Management of Patients With Bipolar Disorder: consensus and controversies. Bipolar Disord. 2005;7(suppl 3):5-69. PubMed CrossRef
15. Kennedy SH, Lam RW, McIntyre RS, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults With Major Depressive Disorder, section 3: pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. PubMed CrossRef
16. United Kingdom National Institute for Health and Care Excellence. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. 2008;CG72. https://www.nice.org.uk/guidance/cg72. Accessed October 29, 2017.
17. United Kingdom National Institute for Health and Care Excellence. Depression in Adults: Recognition and Management. 2009;CG90. https://www.nice.org.uk/guidance/cg90. Accessed October 29, 2017.
18. United Kingdom National Institute for Health and Care Excellence. Bipolar Disorder: Assessment and Management. 2014;CG185. https://www.nice.org.uk/guidance/cg185/resources/bipolar-disorder-assessment-and-management-pdf-35109814379461. Accessed October 29, 2017.
19. United Kingdom National Institute for Health and Care Excellence. Psychosis and Schizophrenia in Adults: Prevention and Management. 2014;CG178. https://www.nice.org.uk/guidance/cg178/resources/psychosis-and-schizophrenia-in-adults-prevention-and-management-pdf-35109758952133. Accessed October 29, 2017.
20. US Department of Veterans Affairs and Department of Defense. Clinical Practice Guideline for Management of Bipolar Disorder in Adults. https://www.healthquality.va.gov/bipolar/bd_306_sum.pdf. 2010.
21. US Department of Veterans Affairs and Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders, version 3.0. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf. 2015.
22. US Department of Veterans Affairs and Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Major Depressive Disorder, version 3.0. https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf. 2016. Accessed October 29, 2017.
23. US Department of Defense. Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder, version 3.0. https://www.guidelinecentral.com/summaries/vadod-clinical-practice-guideline-for-the-management-of-posttraumatic-stress-disorder-and-acute-stress-disorder/#section-society. Accessed October 29, 2017.
24. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267-272. PubMed CrossRef
25. Marder SR, Essock SM, Miller AL, et al. The Mount Sinai Conference on the Pharmacotherapy of Schizophrenia. Schizophr Bull. 2002;28(1):5-16. PubMed CrossRef
26. American Society for Addiction Medicine. National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. 2015. https://www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf?sfvrsn=96df6fc2_24. Accessed October 29, 2017.
27. Expert Group. ‘ Schizophrenia and Diabetes 2003’ Expert Consensus Meeting, Dublin, 3-4 October 2003: consensus summary. Br J Psychiatry suppl. 2004;47:S112-S114. PubMed
28. Firestone A. Diabetes, psychotic disorders and antipsychotic therapy: a consensus statement. Med J Aust. 2005;182(6):310, author reply 310. PubMed
29. United States Food and Drug Administration. FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. 2012. https://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Accessed October 29, 2017.
30. Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348. PubMed CrossRef
31. Cooper SJ, Reynolds GP, Barnes T, et al; With expert co-authors (in alphabetical order). BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol. 2016;30(8):717-748. PubMed CrossRef
32. Beach SR, Celano CM, Noseworthy PA, et al. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1-13. PubMed CrossRef
33. Abnormal IMS. (117-AIMS). In: Guy W, ed. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976:534-537.
34. Institute of Medicine of the National Academies. Committee on Identifying and Preventing Medication Errors. In: Aspden P, Wolcott JA, Bootman JL, et al, eds. Preventing Medication Errors. Washington, DC: The National Academies Press; 2007.
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top