Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
Drug-induced long QT syndrome (LQTS) can be life-threatening and increase the risk of ventricular tachycardia, specifically torsades de pointes (TdP). There are several risk factors for LQTS including female sex, personal history of drug-induced LQTS, family history of congenital LQTS, hypokalemia, structural heart disease, bradycardia, atrioventricular block, concomitant use of QT-prolonging agents, and use of certain medications.
Zolpidem is a nonbenzodiazepine benzodiazepine receptor agonist (nBBRA) used to treat insomnia. There is 1 previous case report4 of LQTS resulting from concurrent zolpidem and amiodarone use.

QTc Prolongation Secondary to Zolpidem Use
Drug-induced long QT syndrome (LQTS) can be life-threatening and increase the risk of ventricular tachycardia, specifically torsades de pointes (TdP). There are several risk factors for LQTS including female sex, personal history of drug-induced LQTS, family history of congenital LQTS, hypokalemia, structural heart disease, bradycardia, atrioventricular block, concomitant use of QT-prolonging agents, and use of certain medications.1-3
Zolpidem is a nonbenzodiazepine benzodiazepine receptor agonist (nBBRA) used to treat insomnia. There is 1 previous case report4 of LQTS resulting from concurrent zolpidem and amiodarone use. Laboratory studies have shown that zolpidem inhibits hERG potassium channels and prolongs action potential duration in human-induced pluripotent stem cell-derived cardiomyocytes, increasing the risk of LQTS.5,6 We report a case of LQTS in the setting of zolpidem extended-release (ER) preparation in a patient with underlying right bundle branch block and prolonged QRS interval, with near-normalization of the QTc after stopping the medication.
Case Report
A 72-year-old woman with a past medical history significant for remote Roux-en-Y gastric bypass surgery, hypothyroidism, mild intermittent asthma that was well controlled, posttraumatic stress disorder, and major depressive disorder was taking zolpidem ER at a dose of 12.5 mg at bedtime for a longstanding history of chronic insomnia disorder. She presented to her primary care provider after an episode of atypical chest pain following an upper respiratory infection. Initial electrocardiogram (ECG) showed a QT corrected interval (QTc) of 503 ms (99th percentile in postpubertal females: 480 ms).7 At that point, the patient was taking buspirone 30 mg/d, levothyroxine 150 mcg/d, mirtazapine 30 mg/d, nortriptyline 50 mg/d, trazodone 100 mg/d, and zolpidem ER 12.5 mg/d, as well as inhaled albuterol 90 mcg as needed and calcium, vitamin B12, and D3 supplements. Over the next 8 months, most medications were gradually tapered and discontinued; the only medications that she continued were levothyroxine and zolpidem ER. During the period of the medication taper, the patient had 4 ECGs, all showing a QTc of over 500 ms.
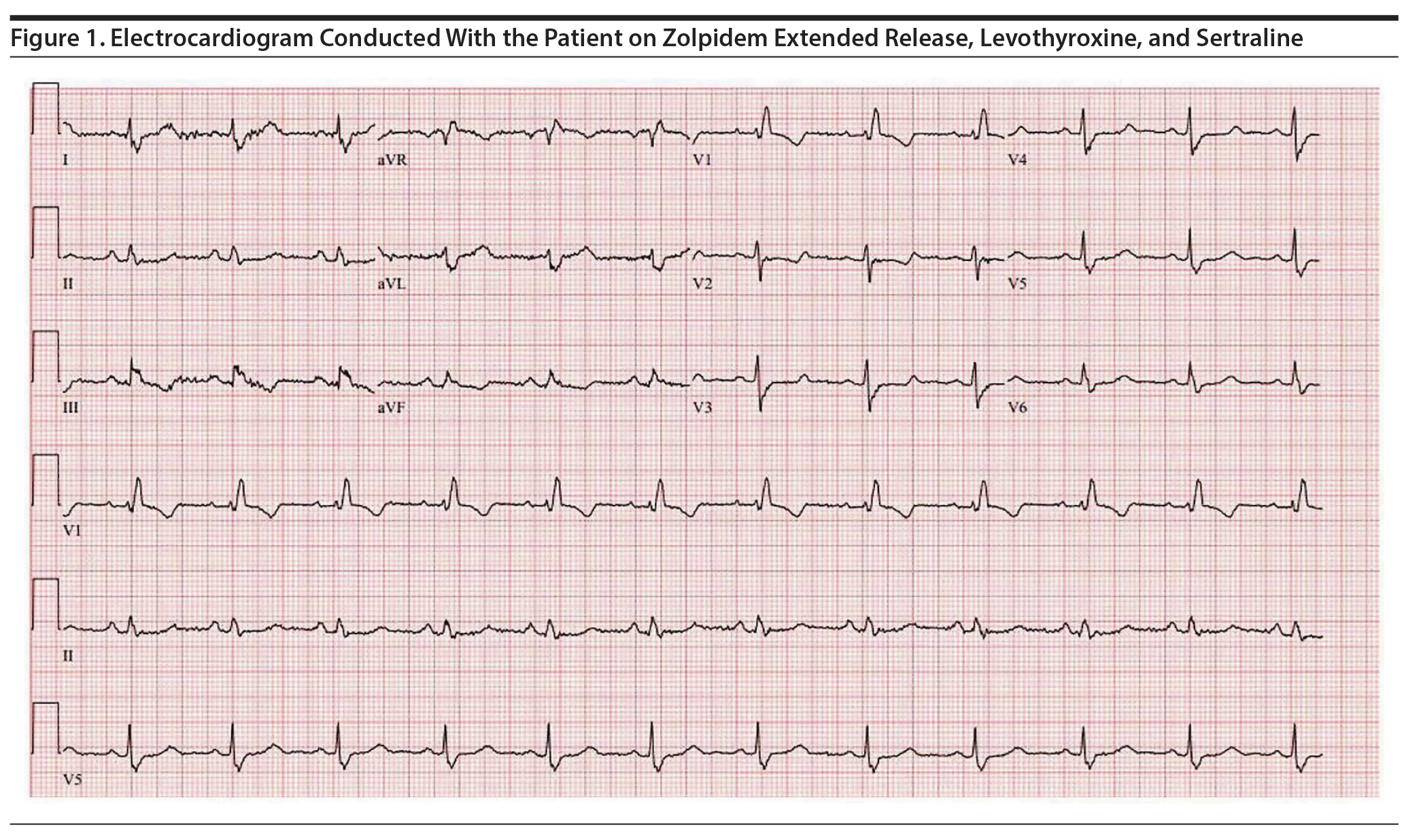
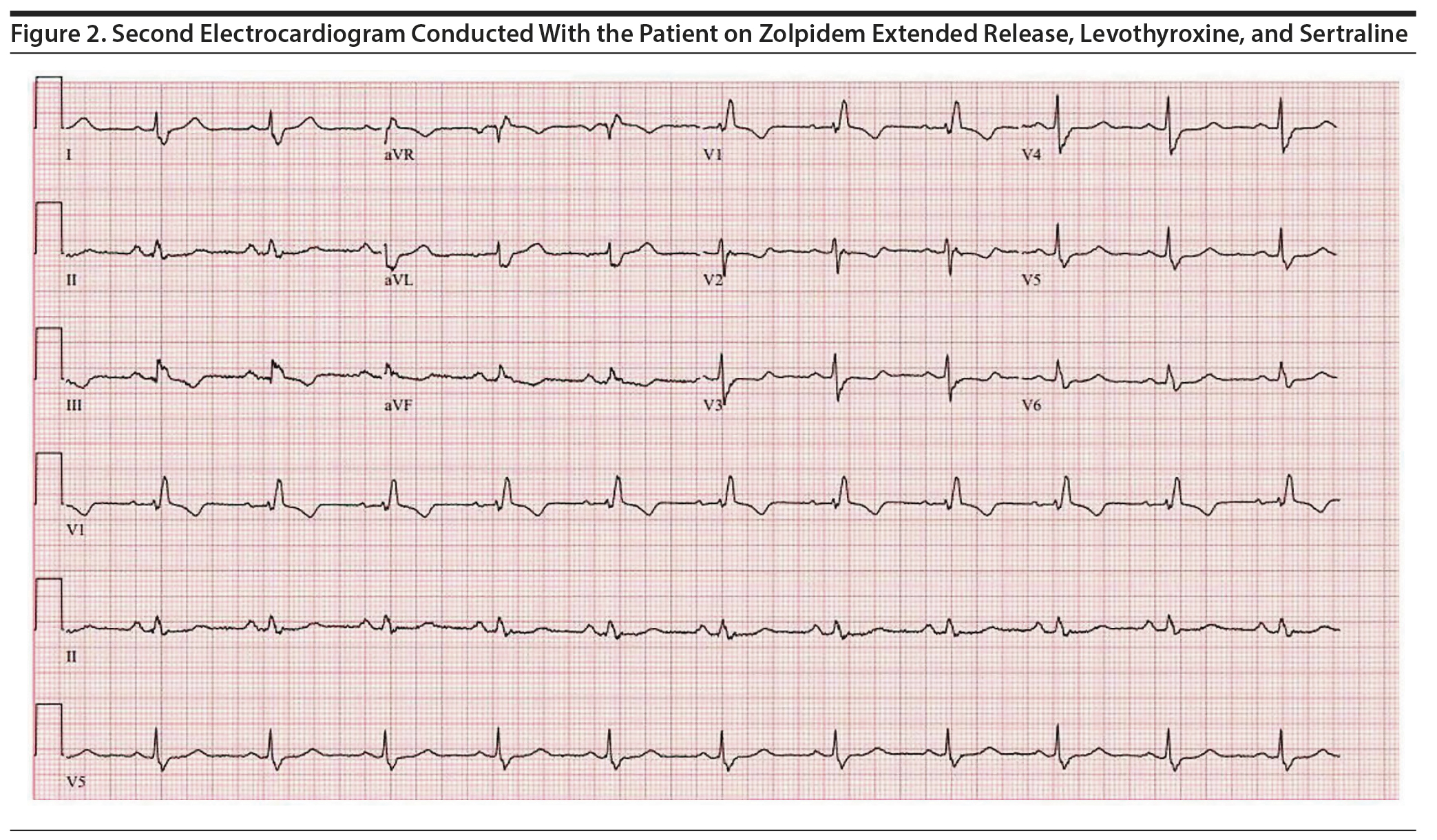
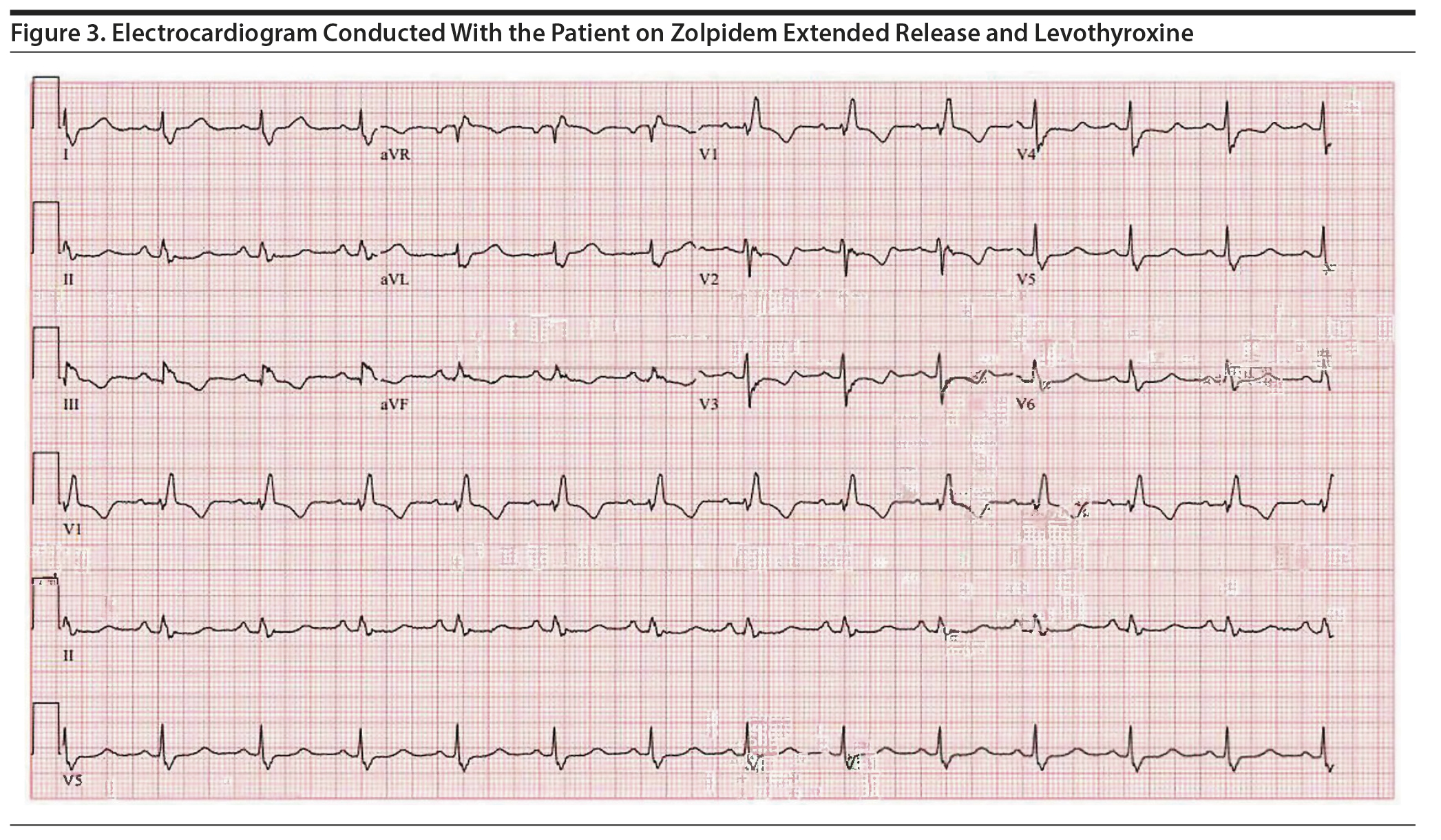
Approximately 4 months later, the patient restarted treatment for depression with sertraline titrated up to a dose of 75 mg/d; follow-up ECG showed a QTc of 506 ms (Figure 1). The patient reported palpitations and was referred to a cardiology specialist who found no abnormalities on physical examination. Holter monitoring, echocardiography, serum electrolytes, thyroid-stimulating hormone, and cardiac catheterization results were unremarkable. The cardiologist recommended continuing levothyroxine, zolpidem ER, and sertraline despite a prolonged QTc of 495 ms given the possibility that the QT was lengthened secondary to right bundle branch block with a prolonged QRS interval (138 ms) (Figure 2). However, 5 months later, a repeat ECG showed a QTc of 507 ms, and thus sertraline was discontinued with a plan to repeat the ECG in 10 days. The repeat ECG showed a QTc of 536 ms (Figure 3) with the patient taking only zolpidem ER and levothyroxine.
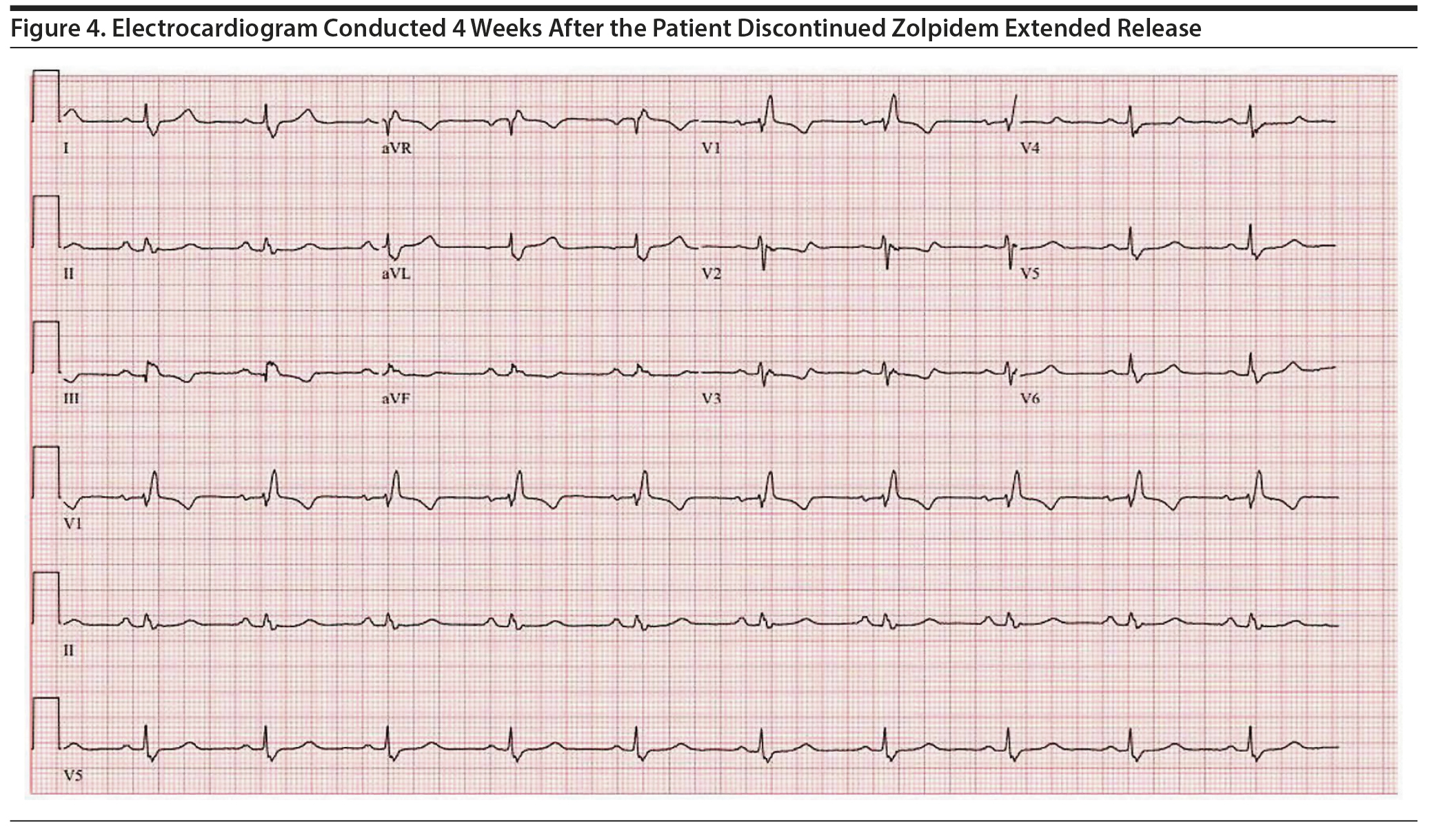
Due to ongoing concerns regarding the QTc, the patient was referred to a sleep medicine specialist, who recommended cognitive-behavioral therapy for insomnia (CBT-I). After completion of 2 sessions, zolpidem ER was tapered and discontinued. Approximately 4 weeks after the discontinuation of zolpidem ER, a follow-up ECG showed a QTc of 481 ms (Figure 4). Additionally, the patient no longer experienced palpitations and had a favorable response to CBT-I with resolution of chronic insomnia.
Discussion
The previous case4 showing an association between zolpidem and LQTS described a 67-year-old woman with a history of congenital heart failure and a prosthetic mitral valve who took zolpidem and amiodarone, resulting in a QTc of 565 ms and TdP. The QTc shortened after discontinuation of both amiodarone and zolpidem.4 One important consideration is the use of the Bazett formula (QTcB = QT/RR1/2) for rate correction.8 This formula continues to be the most utilized method for QT correction but has been shown to have the highest variability, with heart rates above 60 bpm resulting in overcorrection.9-11
Pharmacotherapy for chronic insomnia disorder is common, even in the elderly, and often includes the use of nBBRAs.12,13 There are now 2 cases to suggest that zolpidem can worsen LQTS. In addition, there is vitro research demonstrating the mechanism whereby zolpidem could contribute to a prolonged QTc and increase the risk of TdP.5 This case stresses the importance of conducting future research examining this potential risk and suggests that clinicians should be careful when prescribing zolpidem for the management of insomnia, especially when patients are at risk for LQTS. CBT-I should be considered first-line treatment in most patients with chronic insomnia disorder.14
Published online: March 19, 2020.
Potential conflicts of interest: None.
Funding/support: None.
Additional information: This work was performed at the Mayo Clinic, Rochester, Minnesota.
Patient consent: Consent was obtained from the patient to publish the report, and information has been de-identified to protect anonymity.
REFERENCES
1.Gupta A, Lawrence AT, Krishnan K, et al. Current concepts in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am Heart J. 2007;153(6):891-899. PubMed CrossRef
2.Schwartz PJ, Woosley RL. Predicting the unpredictable: drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67(13):1639-1650. PubMed CrossRef
3.Beach SR, Celano CM, Sugrue AM, et al. QT prolongation, torsades de pointes, and psychotropic medications: a 5-year update. Psychosomatics. 2018;59(2):105-122. PubMed CrossRef
4.Letsas KP, Filippatos GS, Kounas SP, et al. QT interval prolongation and torsades de pointes in a patient receiving zolpidem and amiodarone. Cardiology. 2006;105(3):146-147. PubMed CrossRef
5.Jehle J, Ficker E, Wan X, et al. Mechanisms of zolpidem-induced long QT syndrome: acute inhibition of recombinant hERG K(+) channels and action potential prolongation in human cardiomyocytes derived from induced pluripotent stem cells. Br J Pharmacol. 2013;168(5):1215-1229. PubMed CrossRef
6.De Bruin ML, Pettersson M, Meyboom RH, et al. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur Heart J. 2005;26(6):590-597. PubMed CrossRef
7.Schwartz PJ, Ackerman MJ. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013;34(40):3109-3116. PubMed CrossRef
8.Bazett H. An analysis of the time-relations of the electrocardiograms. Heart. 1920;7:353-370.
9.Chiladakis J, Kalogeropoulos A, Arvanitis P, et al. Preferred QT correction formula for the assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2010;21(8):905-913. PubMed
10.Indik JH, Pearson EC, Fried K, et al. Bazett and Fridericia QT correction formulas interfere with measurement of drug-induced changes in QT interval. Heart Rhythm. 2006;3(9):1003-1007. PubMed CrossRef
11.Vandenberk B, Vandael E, Robyns T, et al. Which QT correction formulae to use for QT Monitoring? J Am Heart Assoc. 2016;5(6):e003264. PubMed CrossRef
12.Albrecht JS, Wickwire EM, Vadlamani A, et al. Trends in insomnia diagnosis and treatment among Medicare beneficiaries, 2006-2013. Am J Geriatr Psychiatry. 2019;27(3):301-309. PubMed CrossRef
13.Kaufmann CN, Spira AP, Alexander GC, et al. Trends in prescribing of sedative-hypnotic medications in the USA: 1993-2010. Pharmacoepidemiol Drug Saf. 2016;25(6):637-645. PubMed CrossRef
14.Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504. PubMed
aDepartment of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota
bCenter for Sleep Medicine, Mayo Clinic, Rochester, Minnesota
*Corresponding author: Bhanu Prakash Kolla, MD, MRCPsych, Center for Sleep Medicine, Mayo Clinic, 2nd St SW, Rochester, MN 55905 ([email protected]).
Prim Care Companion CNS Disord 2020;22(2):19l02506
To cite: Suarez L, Kolla BP, Hall-Flavin D, et al. QTc prolongation secondary to zolpidem use. Prim Care Companion CNS Disord. 2020;22(2):19l02506.
To share: https://doi.org/10.4088/PCC.19l02506
© Copyright 2020 Physicians Postgraduate Press, Inc.
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top