Objective: Executive function (EF) deficits are not generally considered synonymous with attention-deficit/hyperactivity disorder (ADHD). Evidence suggests stimulants improve ADHD symptoms and EF deficits in adults with ADHD, but the relationships between improvements in these domains have not been studied.
Methods: These post hoc analyses used data from a 10-week double-blind, placebo-controlled study of adults with ADHD and EF deficits treated with lisdexamfetamine dimesylate (30-70 mg) or placebo conducted from May 2010 to November 2010. Efficacy endpoints included change from baseline at week 10/early termination (ET) in self-report Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) Global Executive Composite (GEC) T-score and ADHD-Rating Scale with Adult Prompts total score (ADHD-RS-AP-TS). Relationships between ADHD symptom and EF changes were examined using recursive path analyses.
Results: Mediation proportions were 0.62 (indirect and total treatment effect coefficients [95% CI]: -6.85 [-9.83 to -3.86] and -11.12 [-14.88 to -7.37]) for self-report BRIEF-A GEC T-score change from baseline at week 10/ET on ADHD-RS-AP-TS change from baseline at week 10/ET and 0.93 (indirect and total treatment effect coefficients [95% CI]: -10.34 [-14.11 to -6.57] and -11.18 [-15.80 to -6.55]) for ADHD-RS-AP-TS change from baseline at week 10/ET on self-report BRIEF-A GEC T-score change from baseline at week 10/ET.
Conclusions: Although these data suggest ADHD symptom and EF deficit improvement following lisdexamfetamine are interdependent, it is advantageous to use measures like the BRIEF-A to assess stimulant effects on the wide range of EF deficits associated with ADHD that are not captured by the ADHD-RS-AP alone.
Trial Registration: Data used in this secondary analysis came from ClinicalTrials.gov identifier: NCT01101022.
Relationships Between Executive Function Improvement and ADHD Symptom Improvement With Lisdexamfetamine Dimesylate in Adults With ADHD and Executive Function Deficits:
A Post Hoc Analysis
ABSTRACT
Objective: Executive function (EF) deficits are not generally considered synonymous with attention-deficit/hyperactivity disorder (ADHD). Evidence suggests stimulants improve ADHD symptoms and EF deficits in adults with ADHD, but the relationships between improvements in these domains have not been studied.
Methods: These post hoc analyses used data from a 10-week double-blind, placebo-controlled study of adults with ADHD and EF deficits treated with lisdexamfetamine dimesylate (30-70 mg) or placebo conducted from May 2010 to November 2010. Efficacy endpoints included change from baseline at week 10/early termination (ET) in self-report Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) Global Executive Composite (GEC) T-score and ADHD-Rating Scale with Adult Prompts total score (ADHD-RS-AP-TS). Relationships between ADHD symptom and EF changes were examined using recursive path analyses.
Results: Mediation proportions were 0.62 (indirect and total treatment effect coefficients [95% CI]: -6.85 [-9.83 to -3.86] and -11.12 [-14.88 to -7.37]) for self-report BRIEF-A GEC T-score change from baseline at week 10/ET on ADHD-RS-AP-TS change from baseline at week 10/ET and 0.93 (indirect and total treatment effect coefficients [95% CI]: -10.34 [-14.11 to -6.57] and -11.18 [-15.80 to -6.55]) for ADHD-RS-AP-TS change from baseline at week 10/ET on self-report BRIEF-A GEC T-score change from baseline at week 10/ET.
Conclusions: Although these data suggest ADHD symptom and EF deficit improvement following lisdexamfetamine are interdependent, it is advantageous to use measures like the BRIEF-A to assess stimulant effects on the wide range of EF deficits associated with ADHD that are not captured by the ADHD-RS-AP alone.
Trial Registration: Data used in this secondary analysis came from ClinicalTrials.gov identifier: NCT01101022.
Prim Care Companion CNS Disord 2020;22(3):19m02559
To cite: Brown TE, Chen J, Robertson B. Relationships between executive function improvement and ADHD symptom improvement with lisdexamfetamine dimesylate in adults with ADHD and executive function deficits: a post hoc analysis. Prim Care Companion CNS Disord. 2020;22(3):19m02559.
To share: https://doi.org/10.4088/PCC.19m02559
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Keck School of Medicine, University of Southern California, Los Angeles, and Brown Clinic for Attention & Related Disorders, Manhattan Beach, California
bBiostatistics, Shire, a member of the Takeda group of companies, Lexington, Massachusetts
cGlobal Clinical Development, Shire, a member of the Takeda group of companies, Lexington, Massachusetts; currently employed by Yumanity Therapeutics Inc, Cambridge, Massachusetts
*Corresponding author: Thomas E. Brown, PhD, Brown Clinic for Attention & Related Disorders, 500 S Sepulveda Blvd, Ste 218, Manhattan Beach, CA 90266 ([email protected]).
The understanding of attention-deficit/hyperactivity disorder (ADHD) has evolved over the past decade, with substantial clinical and empirical research suggesting diagnostic criteria for ADHD based on previous and current versions of the Diagnostic and Statistical Manual of Mental Disorders (DSM) and measures of executive function (EF) are best used together to more fully understand the impairment, prognosis, and therapeutic needs of patients with ADHD. Although it is widely accepted that ADHD is a complex syndrome, impairment of central cognitive management systems, specifically EF and related activities, appears to result in some of the most impairing and predictive features of ADHD.1-4 EF refers to activities carried out by brain circuitry that prioritize, integrate, and regulate other cognitive activities. Gioia et al5 defined EF as processes responsible for purposeful, goal-directed, problem-solving behavior, such as those that manage, guide, and direct cognitive, emotional, and behavioral functions. As noted by Vohs and Baumeister,6 EF provides a mechanism for self-regulation. Depending on the criteria used to define EF impairment, approximately 30% to > 70% of adults diagnosed with ADHD based on the DSM, Fourth Edition (DSM-IV) exhibit some level of EF deficit.7,8
Barkley et al,9 Biederman et al,10 Faraone et al,11 and Kessler et al12 have presented data demonstrating that combining specific types of EF impairments with DSM-based ADHD diagnostic criteria provides a more adequate ADHD assessment than do the DSM ADHD criteria alone. In a study assessing 2 national community samples of adults who were screened for ADHD using both EF measures and DSM-IV symptom criteria, Kessler et al12 reported that a factor analysis indicated a 3-factor structure for adult ADHD. One of the factors included 6 non-DSM symptoms related to EF (difficulty planning, prioritizing, multitasking, remembering details, meeting deadlines, and maintaining self-discipline) and 3 DSM symptoms related to EF (difficulty organizing tasks, makes careless mistakes, and loses things). Further, logistic regression analyses demonstrated that EF deficits (ie, difficulty prioritizing work, trouble planning ahead, cannot complete tasks on time) were discriminating predictors of adult ADHD.12 On the basis of these findings and others, the authors concluded that problems associated with EF are present among almost all adults with ADHD.12,13 Additionally, the symptoms of adult EF had no significant comorbidities with other DSM-IV disorders and therefore can be used to differentiate other conditions from ADHD.12 In support of these findings, a validation of the Adult ADHD Self-Report Scale based on the DSM, Fifth Edition (DSM-5 ASRS), included 2 non-DSM-5 EF symptoms (puts things off to last minute, depends on others to keep life in order) in a 6-item screener.14 Furthermore, in an analysis of the relationship between the core ADHD symptoms as defined by the DSM-5 and EF, it was demonstrated that both the inattentive and hyperactive/impulsive symptoms of ADHD were significantly correlated with and highly predictive of EF deficits.15
Although there is an emerging consensus on the important role of EF in mediating ADHD symptoms, there is lack of agreement on the role of self-report EF scales in diagnosing ADHD or an appreciation of how the relationship between EF and DSM-defined ADHD symptoms may affect treatment decisions or patient outcomes. Although some assume EF impairments must be measured with classical tests of EF, there is support for using normed rating scales that elicit information about the effectiveness of an individual’s EF for multiple tasks of daily life in a variety of settings over time.2,16-20 Furthermore, some studies10,13 suggest EF rating scales are better than neuropsychological tests of EF for assessing impairment of major life activities. In a study13 comparing neuropsychological tests of EF with self-reported EF rating scales, self-report rating scales were significantly more effective in predicting impairment on multiple measures of occupational functioning. Another study10 of individuals with ADHD and EF deficits (and full-scale intelligence quotients ≥ 70) provided additional support for using self-report rating scales to assess ADHD-related impairments of EF. In this study,10 neuropsychological tests primarily identified participants with lower IQ and achievement test scores, while self-report EF measures identified individuals with more severe ADHD symptoms, higher frequencies of psychiatric comorbidities, and greater levels of interpersonal deficits. All of the studies of EF and ADHD cited previously, including Barkley et al,9 Biederman et al,10 Faraone et al,11 and Kessler et al,12 utilized normed rating scales, not neuropsychological tests. Thus, the data obtained from these studies support the concept that ADHD assessment is done most effectively when using both DSM diagnostic criteria and self-report measures of EF.
Although studies21-27 have examined various relationships between EF and ADHD, the effect of ADHD pharmacotherapy on DSM-based ADHD symptoms and on EF measured using normed self-report EF rating scales in adults with DSM-IV Text Revision (DSM-IV-TR)-based ADHD has not been systematically studied. In a published study of adults with ADHD and clinically relevant EF deficits, defined as self-report Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) Global Executive Composite (GEC) T-scores ≥ 65, lisdexamfetamine dimesylate produced significantly greater EF improvement than placebo. At treatment week 10/early termination (ET), least square (LS) mean BRIEF-A GEC T-score changes from baseline (primary efficacy endpoint) were -22.3 with lisdexamfetamine and -11.1 with placebo, representing an effect size of 0.74.28 In the same study,28 LS mean ADHD-Rating Scale with Adult Prompts (ADHD-RS-AP) total score reductions from baseline at week 10/ET (secondary efficacy endpoint) were -21.4 with lisdexamfetamine and -10.3 with placebo, representing an effect size of 0.94. However, the relationship between ADHD symptom improvement and EF improvement in this study was not investigated, and no such analyses have been conducted in other ADHD pharmacotherapy studies.
Given the association of EF with adult ADHD,12,29 it could be hypothesized that the effects of lisdexamfetamine on ADHD symptoms and EF in the aforementioned study28 were interdependent. This article reports post hoc analyses of data from the aforementioned lisdexamfetamine study.28 These analyses were conducted to assess the reciprocal relationships between the effects of lisdexamfetamine on EF, as measured by the normed BRIEF-A self-report and informant report, and DSM-IV-TR-based ADHD symptoms, as measured by ADHD-RS-AP score change. Specifically, path analyses were used to examine (1) how improvement in EF following lisdexamfetamine mediates improvement in ADHD symptoms and, conversely, (2) how improvement in ADHD symptoms following lisdexamfetamine mediates improvement in EF.
METHODS
The overall methodology of this phase 4 study has previously been described.28 Key information related to the study design and participants is summarized in the following sections.
Study Design and Treatment Regimen
This randomized, double-blind, placebo-controlled study (ClinicalTrials.gov identifier: NCT01101022) enrolled adults with ADHD and clinically relevant EF deficits from 33 clinical research sites in the United States between May 2010 and November 2010. The study was conducted in accordance with the guidelines of the International Conference on Harmonization of Good Clinical Practice and the principles of the 18th World Medical Assembly, including amendments of the 29th, 35th, 41st, and 48th World Medical Assemblies and the Declaration of Helsinki. The protocol and relevant supporting materials were reviewed and approved by local institutional review boards and appropriate regulatory agencies before study initiation. Written informed consent was required from participants before any procedures were conducted.
CLINICAL POINTS
- Impairment of executive function is increasingly recognized as a useful elaboration of the diagnostic criteria for attention-deficit/hyperactivity disorder (ADHD) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM).
- Self-report rating scales, such as the Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A), can provide an effective normed measure of executive function impairments associated with ADHD.
- Use of a normed self-report scale, such as the BRIEF-A, in combination with DSM criteria for ADHD can provide a useful way to identify patients with ADHD, to monitor the effectiveness of ADHD treatment for a range of symptoms, and to provide guidance for increasing the effectiveness of ADHD treatment.
Following a screening and washout period, eligible participants were randomized 1:1 to dose-optimized lisdexamfetamine or to placebo capsules that were identical in appearance to lisdexamfetamine capsules. Participants then entered a 10-week double-blind treatment phase (4 weeks of dose optimization, followed by 6 weeks of dose maintenance). During the 4-week dose-optimization period, lisdexamfetamine treatment was initiated at 30 mg during week 1. The dose was then increased to 50 mg during treatment week 2 and 70 mg during treatment week 3. A single dose reduction was permitted during dose optimization. During the 6-week dose-maintenance period, participants were maintained on the optimized lisdexamfetamine dose established during dose optimization; no further dose reductions were allowed. A participant’s optimal dose was defined as one associated with a ≥ 30% decrease in ADHD-RS-AP total score from baseline and a Clinical Global Impressions-Improvement rating of 1 or 2, with acceptable tolerability.
Participants
Men or women (aged 18-55 years) meeting DSM-IV-TR criteria for a primary ADHD diagnosis were eligible to participate. Participants were required to have baseline self-report BRIEF-A GEC T-scores ≥ 65 and baseline ADHD-RS-AP total scores ≥ 28. Participants were also required to have a close domicile relationship (eg, spouse or significant other) for ≥ 6 months before screening to ensure availability of an informant able to report the participant’s behavior and symptoms.
Individuals were excluded if they had comorbid psychiatric conditions, including severe Axis I or II disorders, controlled with prohibited medications or uncontrolled and associated with significant symptoms. Additional exclusion criteria included having a body mass index < 18.5 kg/m2 or ≥ 40 kg/m2; being considered a suicide risk, having previously made a suicide attempt, or currently demonstrating active suicidal ideation; having a history of cardiovascular disease or any serious cardiac issue that could increase vulnerability to the sympathomimetic effects of a psychostimulant; having a history of moderate to severe hypertension, resting sitting systolic blood pressure > 139 mm Hg, or diastolic blood pressure > 89 mm Hg; having ADHD that was well controlled by current therapy; or previously failing to respond to an adequate course of amphetamine treatment.
Endpoints
The primary efficacy endpoint was change from baseline at week 10/ET in self-report BRIEF-A GEC T-score. The BRIEF-A is a validated 75-item instrument measuring aspects of EF in everyday life during the past month; both self-report and informant-report forms are available.30 The 75 items of the BRIEF-A form 9 clinical scales from which a GEC score can be generated as a summary measure that incorporates all clinical scales. Scores on the BRIEF-A are reported as T-scores, which are linear transformations that normalize raw scores to a standardization sample; the normative mean of the standardization sample is 50 (SD = 10).30 The inclusion criterion defining impaired EF in this study (GEC T-score ≥ 65) is 1.5 SD above the normative sample mean (higher T-scores indicate more severe impairment).30 The BRIEF-A (self-report and informant-report) was assessed at screening, baseline, and treatment weeks 4, 7, and 10/ET.
Change from baseline at week 10/ET on the ADHD-RS-AP was a secondary efficacy endpoint. The 18-item ADHD-RS-AP is a validated scale based on the ADHD-RS, which was developed for children,31 that employs adult prompts to allow clinicians to probe the extent, frequency, breadth, severity, and consequences of ADHD symptoms in adults based on DSM-IV-TR diagnostic criteria.32,33 Each item is scored on a 4-point scale ranging from 0 (no symptoms) to 3 (severe symptoms), with total scores ranging from 0 to 54. The scale is divided into 2 subscales (hyperactivity/impulsivity [H/I] and inattentiveness [IA]), which each include 9 items. The ADHD-RS-AP was assessed at screening, baseline, treatment weeks 1 through 4, and treatment week 10/ET.
Data Analysis and Statistics
A complete reporting of the prespecified analyses and results from this study have been described elsewhere.28 These post hoc analyses used simple recursive path analyses in a classical mediation model to assess the relationships between changes from baseline to week 10/ET in BRIEF-A GEC T-scores and ADHD-RS-AP scores. Simple recursive path analyses involving treatment (lisdexamfetamine vs placebo), mediators, and outcomes (self-report BRIEF-A GEC T-scores, informant-report T-scores, ADHD-RS-AP total scores, ADHD-RS-AP subscale scores) were conducted, with direct arrows from the treatment group, mediator baseline, and outcome baseline to the mediator and the outcome and from the mediator to the outcome. Path analyses are best suited for these examinations because they allow for the decomposition of correlations among variables (eg, the total effect, the direct effect, and the indirect effect via mediation), which enhances the interpretation of relations and the pattern of effects of one variable on another.
The mediation proportion was defined as the indirect treatment effect divided by the total treatment effect. All analyses were conducted using the full analysis set (participants taking ≥ 1 study drug dose in the double-blind phase and having ≥ 1 postrandomization primary efficacy assessment). These analyses were not included in the prespecified statistical analysis plan for which the study was powered. As such, all reported P values are nominal (uncorrected for multiple comparisons) and are included for descriptive purposes only.
RESULTS
Disposition and Demographics
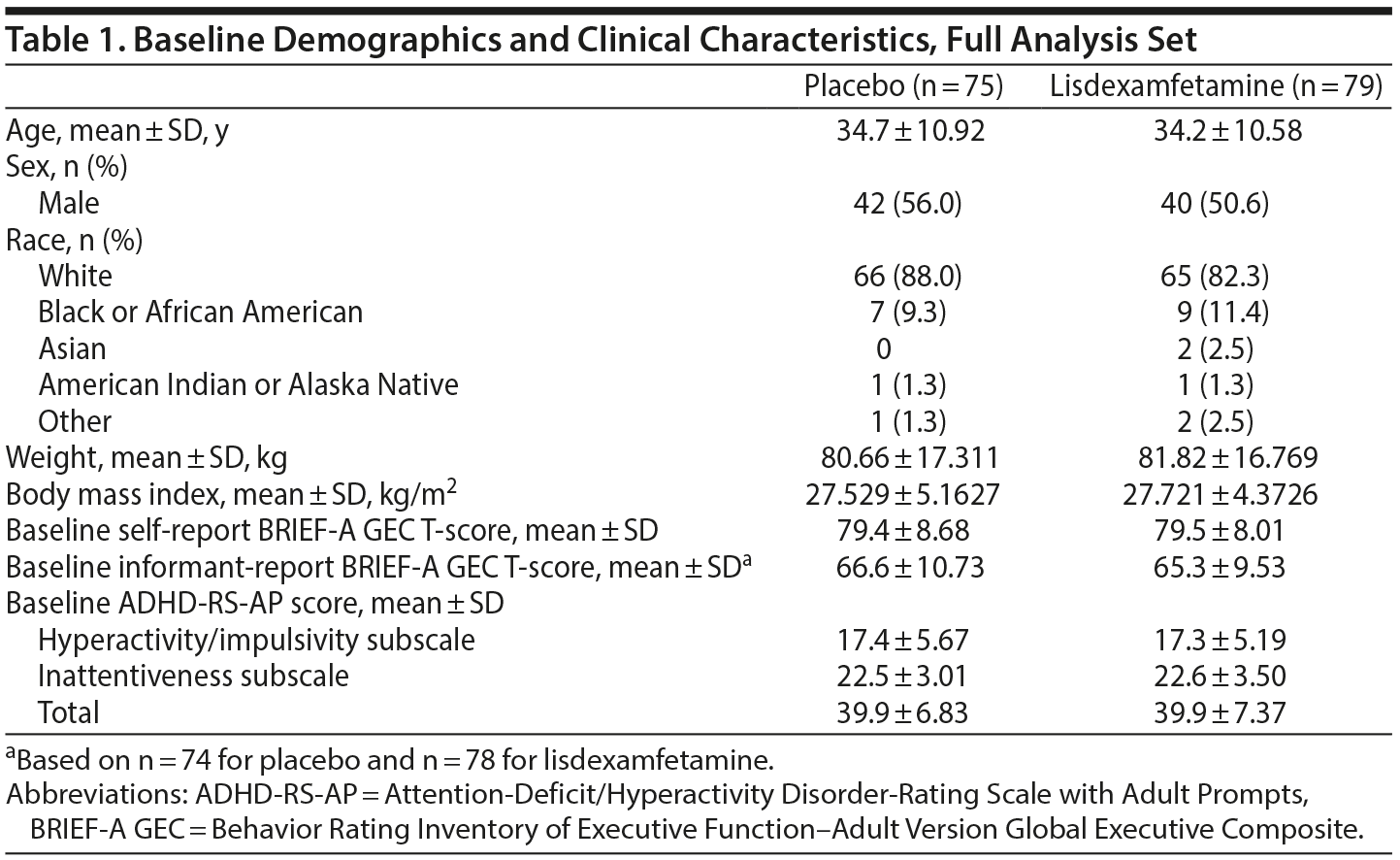
A full description of participant disposition has been published.28 In brief, 161 participants were enrolled and randomized to treatment (placebo, n = 81; lisdexamfetamine, n = 80). The full analysis set included 154 participants (placebo, n = 75; lisdexamfetamine, n = 79). The study was completed by 115 participants (placebo, n = 53; lisdexamfetamine, n = 62).
Baseline demographic and clinical characteristics are presented in Table 1. Most participants were white and slightly more than 50% were men. Overall, participant characteristics were well balanced across treatment arms.
Concomitant medication use was reported in 68.8% (55/80) and 72.2% (57/79) of the placebo and lisdexamfetamine groups, respectively. Medications used by ≥ 5 participants in either group (placebo; lisdexamfetamine) were ibuprofen (16.3% [13/80]; 24.1% [19/79]), acetaminophen (16.3% [13/80]; 12.7% [10/79]), multivitamins (11.3% [9/80]; 21.5% [17/79]), loratadine (3.8% [3/80]; 8.9% [7/79]), and vitamin D (3.8% [3/80]; 8.9% [7/79]).
BRIEF-A GEC T-Score Change (mediator) on ADHD-RS-AP Score Change (outcome)
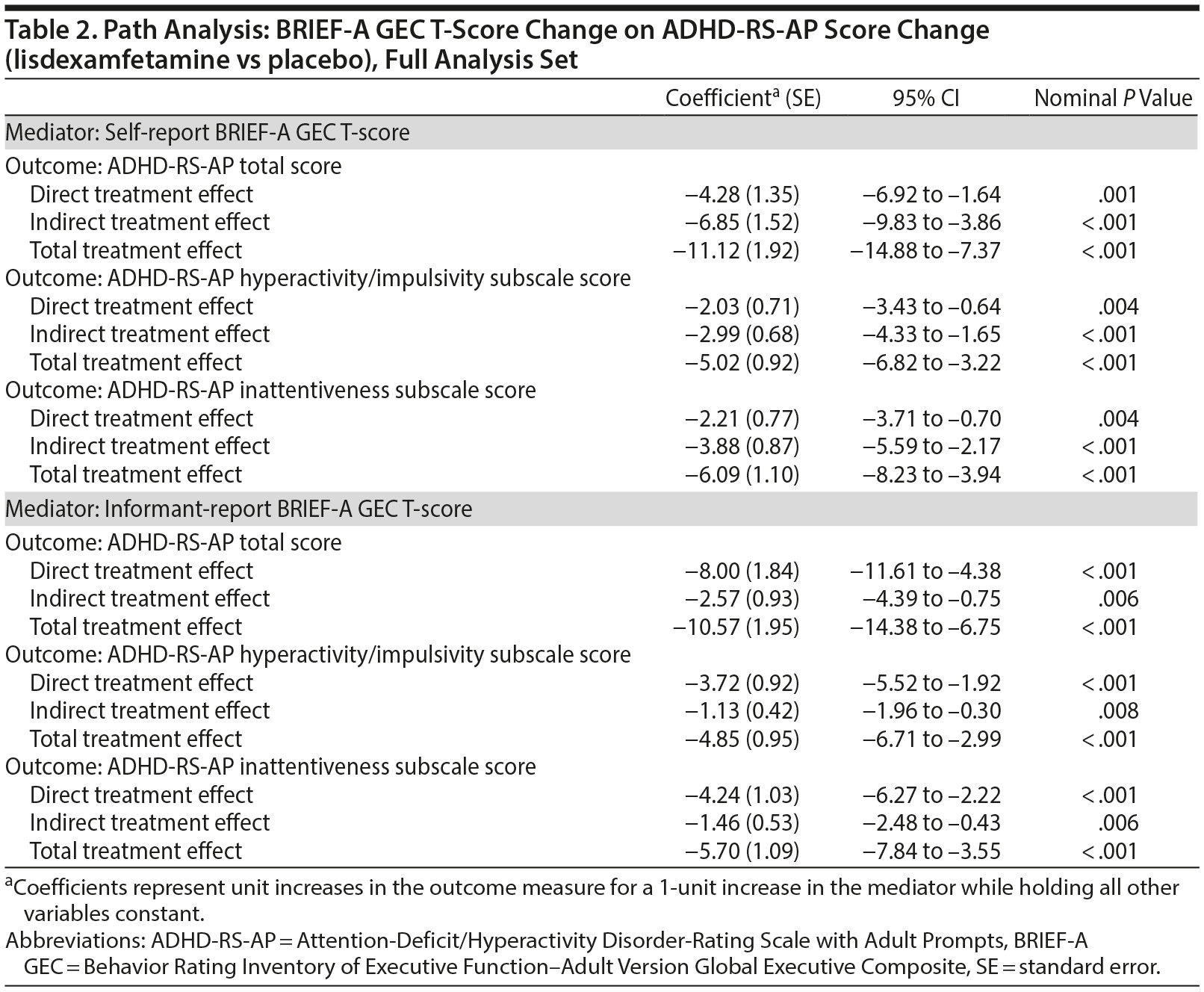
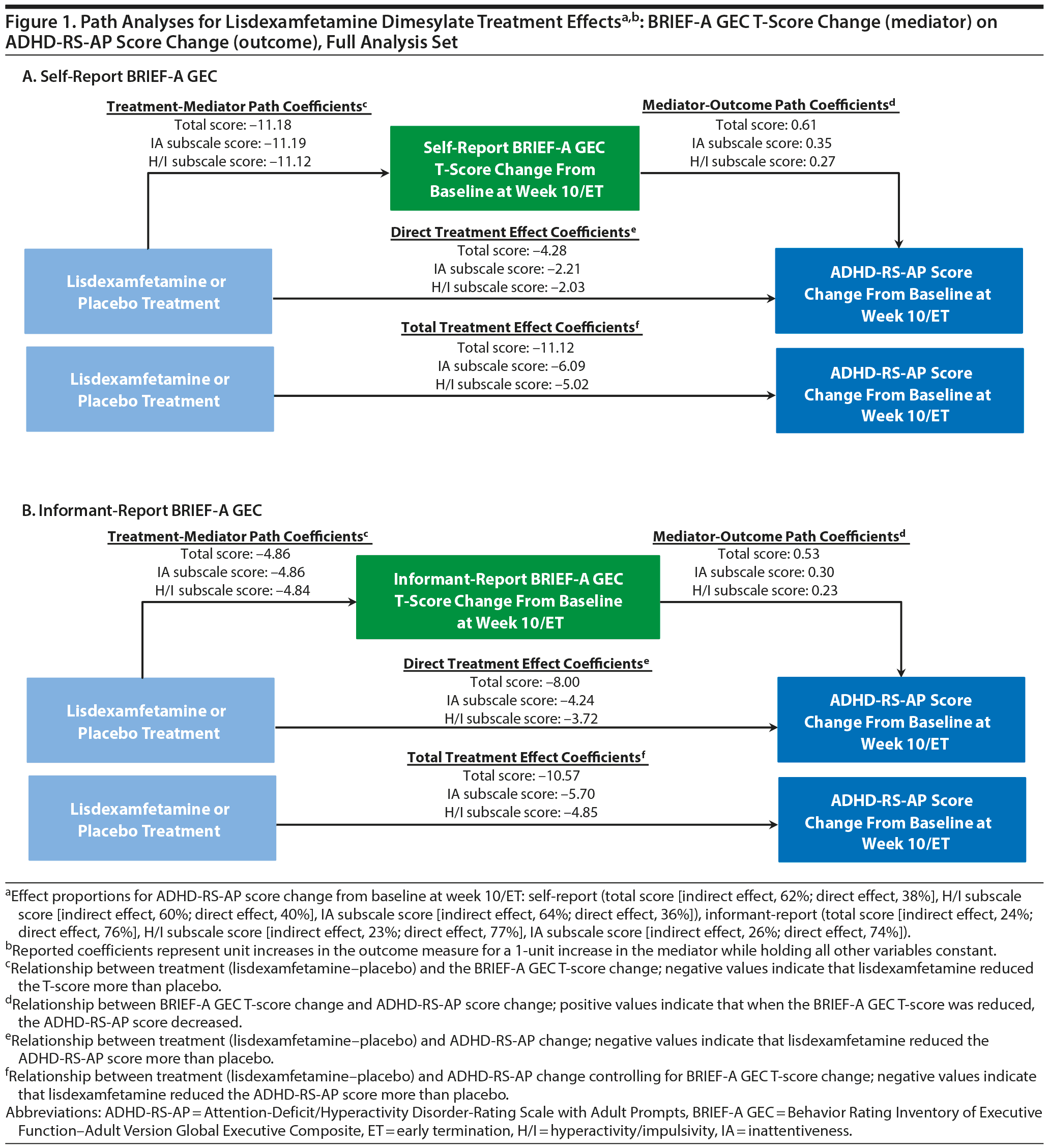
Path analysis results with self-report BRIEF-A GEC T-score change or informant-report BRIEF-A GEC T-score change as the mediator and ADHD-RS-AP score change as the outcome are summarized in Table 2 and Figure 1. The indirect treatment effects of self-report BRIEF-A GEC T-score changes at week 10/ET on ADHD-RS-AP total, H/I, and IA score changes at week 10/ET were statistically significant (all nominal P < .001, Table 2). The mediation proportions of the self-report BRIEF-A GEC T-score change from baseline at week 10/ET on ADHD-RS-AP score change from baseline at week 10/ET were 0.62 for total score, 0.60 for H/I subscale score, and 0.64 for IA subscale score. The indirect treatment effects of informant-report BRIEF-A GEC T-score changes at week 10/ET on ADHD-RS-AP total, H/I, and IA score changes at week 10/ET were statistically significant (all nominal P ≤ .008, Table 2). The mediation proportions of the informant-report BRIEF-A GEC T-score change from baseline at week 10/ET on ADHD-RS-AP score change from baseline at week 10/ET were 0.24 for total score, 0.23 for H/I subscale score, and 0.26 for IA subscale score.
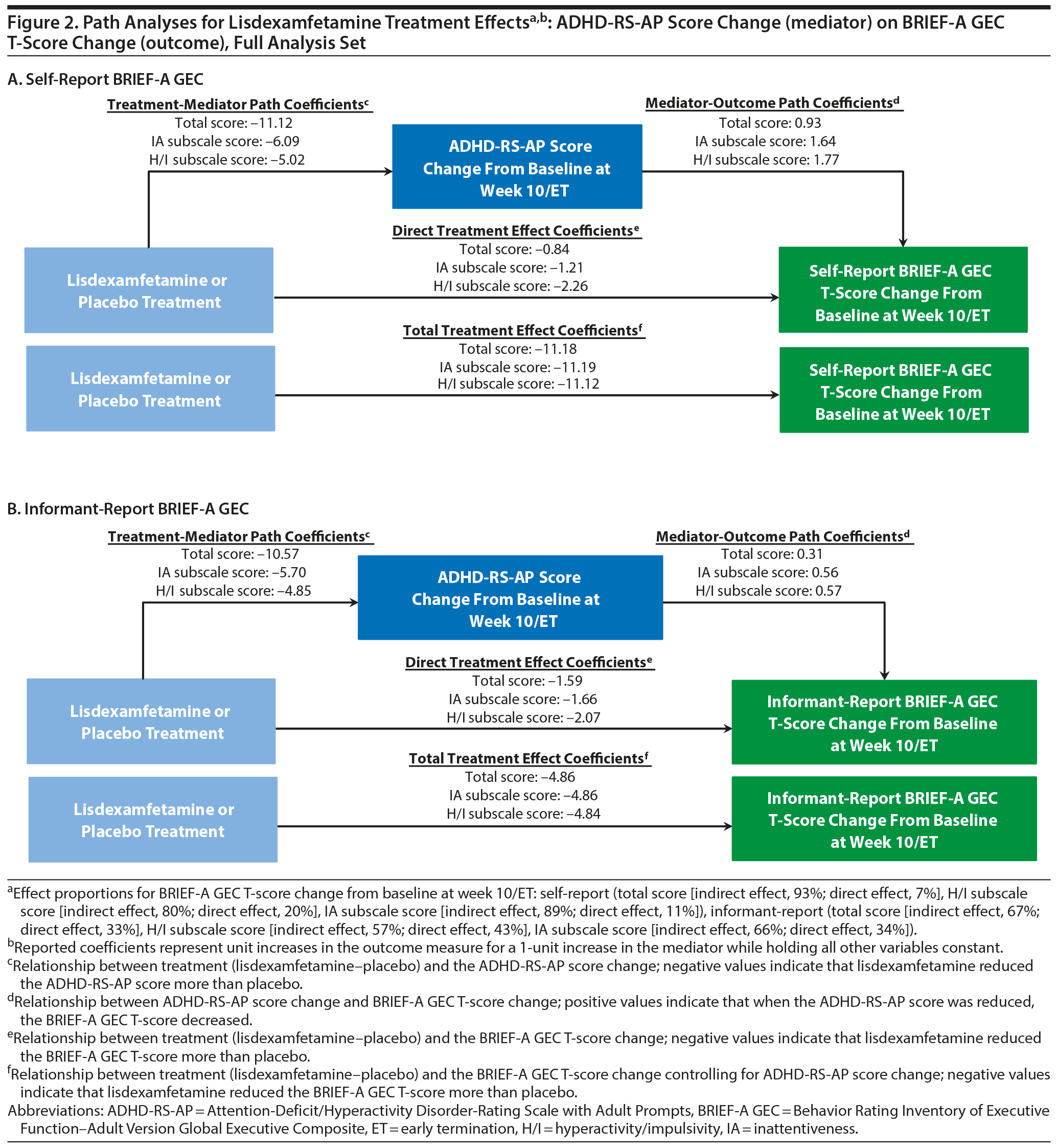
ADHD-RS-AP Score Change (mediator) on BRIEF-A GEC T-Score (outcome)
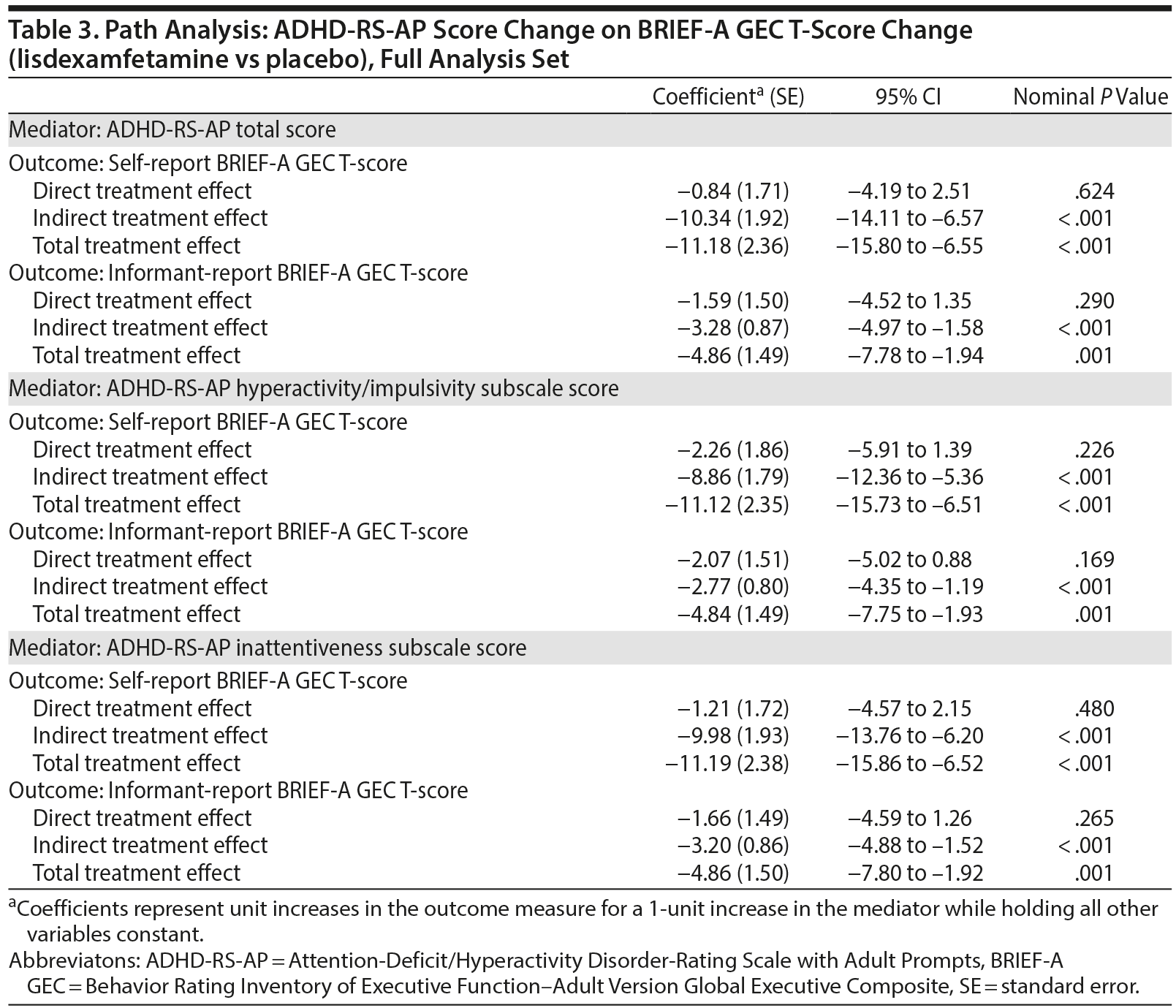
Path analysis results with ADHD-RS-AP score change as the mediator and self-report or informant-report BRIEF-A GEC T-score change as the outcome are summarized in Table 3 and Figure 2. The indirect treatment effects of ADHD-RS-AP total, H/I, and IA score changes on self-report BRIEF-A GEC T-score changes and informant-report BRIEF-A GEC T-score changes at week 10/ET were statistically significant (all nominal P ≤ .001, Table 3). The mediation proportions of the ADHD-RS-AP score changes on self-report BRIEF-A GEC T-score change were 0.93 for ADHD-RS-AP total score, 0.80 for ADHD-RS-AP H/I subscale score, and 0.89 for ADHD-RS-AP IA subscale score. The mediation proportions of the ADHD-RS-AP score changes from baseline at week 10/ET on informant-report BRIEF-A GEC T-score were 0.67 for ADHD-RS-AP total score, 0.57 for ADHD-RS-AP H/I subscale score, and 0.66 for ADHD-RS-AP IA subscale score.
DISCUSSION
These post hoc path analyses demonstrate that EF improvement, measured by self-report and informant-report BRIEF-A GEC T-scores, and DSM-IV-TR-based ADHD symptom improvement, measured by ADHD-RS-AP scores, are interdependent following lisdexamfetamine treatment. Based on the mediation proportions, it was observed that effects of lisdexamfetamine on self-report BRIEF-A GEC T-scores (the mediator) accounted for 62% of the effects of lisdexamfetamine on ADHD-RS-AP total score (the outcome). Conversely, the effects of lisdexamfetamine on ADHD-RS-AP total score (the mediator) accounted for 93% of the effects on BRIEF-A GEC T-score (the outcome). Of note, the indirect mediation proportions for BRIEF-A GEC T-score changes on ADHD-RS-AP score changes were greater for the self-report BRIEF-A than for the informant-report BRIEF-A. Furthermore, the indirect mediation proportions for ADHD-RS-AP score changes on BRIEF-A GEC T-score changes were greater than for the BRIEF-A GEC T-score changes on the ADHD-RS-AP score changes.
Previous publications,34-36 including the primary report from this study,28 have revealed that stimulants can reduce the core symptoms of ADHD and EF deficits in individuals with ADHD. However, to the best of our knowledge, this is first demonstration of an interdependent relationship between concurrent reductions in EF deficits and ADHD symptoms in adults treated with stimulants. These findings are supportive of the hypothesis that EF is a central aspect of ADHD impairment.12,16,20 For example, in a study12 based on data from clinical interviews of subsamples of individuals from the National Comorbidity Replication Survey and from a survey of a large managed health care plan, impaired EF was found to be an important predictor of adult ADHD in individuals meeting full DSM diagnostic criteria for ADHD as children. Importantly, unlike other highly predictive adult ADHD symptoms—all of which involved inattention—impaired EF was not significantly comorbid with other adult DSM-IV disorders, suggesting that EF more specifically differentiated adult ADHD from other adult DSM disorders.12 Furthermore, in the DSM-5 ASRS,14 2 of 6 items included in the screener relate to non-DSM-5 EF symptoms (puts things off to the last minute, depends on others to keep life in order), suggesting that EF is a key component of adult ADHD.
As previously noted, the magnitude of the mediation effect of EF on ADHD symptom reduction was lower when EF was based on informant report than on self-report. The finding that informant ratings did not demonstrate as substantial an impact on treatment as did the self-report data is understandable because informant ratings only provide input on observable behaviors. Informant ratings do not provide adequate information about the participant’s subjective experiences, such as having difficulty in mobilizing attention, being motivated to start tasks, having problems in sustaining focus and effort, and having struggles with recall from working memory. Future studies need to further examine this issue so the reason for this difference can be more clearly identified. Specifically, what factors are identified via self-report that might contribute to the increased mediation effect (eg, improved internal sense of control, time management, or insight associated with improved attention). Further, the insight of individuals with ADHD into their own behavior may be poor, so corroborative evidence from neuropsychological testing or an objective third party could prove to be useful. It is also noteworthy that the magnitude of the mediator effects of ADHD-RS-AP score changes on BRIEF-A GEC T-score changes was greater than the mediator effects of BRIEF-A GEC T-score changes on ADHD-RS-AP score changes.
There are several limitations that should be considered when interpreting these data. First, as is the case for all classical mediation analyses in a randomized trial with a post-baseline mediator, the results are subject to the limitation that there are no additional variables confounding the relationships between treatment and mediator, mediator and outcome, and treatment and outcome. Additional limitations of the path analysis are assumptions that relationships among variables in the model are linear, additive, and causal; that each residual is not correlated with variables that precede it in the model; that causal flow is assumed to be 1 way; that the variables are measured on an interval scale; and that the variables are measured without error. Second, these data are based on post hoc assessments for which the study was not powered. As such, reported P values are nominal and descriptive in nature. Last, these analyses are specific to the enrolled population. Therefore, it cannot be concluded that these results are generalizable to a more heterogeneous population of adults diagnosed with ADHD.
In conclusion, these analyses demonstrate for the first time that concurrent reductions in EF deficits and in DSM-based ADHD symptoms during lisdexamfetamine treatment are interdependent. These findings further emphasize that it is advantageous to use measures like the BRIEF-A to assess the impact of stimulants on the wide range of EF deficits associated with ADHD that are not specifically captured by the ADHD-RS-AP.
Submitted: October 23, 2019; accepted February 14, 2020.
Published online: May 28, 2020.
Potential conflicts of interest: Dr Brown has received research support from Shire; has served as a consultant for Ironshore, NLS, Sunovion, Supernus, and Shire; and has received publication royalties from American Psychiatric Publishing, Jossey-Bass/Wiley, Pearson, Routledge, and Yale University Press. Ms Chen is an employee of Shire, a member of the Takeda group of companies, and holds Takeda stock and/or stock options. Dr Robertson was an employee of Shire, a member of the Takeda group of companies, at the time this research was conducted and holds Takeda stock and/or stock options.
Funding/support: This clinical research was funded by Shire Development LLC, a member of the Takeda group of companies, Lexington, Massachusetts. Shire Development LLC, a member of the Takeda group of companies, provided funding to Complete Healthcare Communications, LLC (CHC; North Wales, Pennsylvania), a CHC Group company, for support in writing and editing this manuscript.
Role of the sponsor: The sponsor was involved in the design and conduct of the data analyses, in the interpretation of the data, and in the preparation, review, and approval of the manuscript. The final decision on the content of the report and on submission of the manuscript to the Primary Care Companion for CNS Disorders was made by the authors.
Previous presentation: Presented at the 2018 annual meeting of the American Professional Society of ADHD and Related Disorders; January 12-14, 2018; Washington, DC ▪ 2018 annual meeting of the US Psychiatric and Mental Health Congress; October 25-28, 2018; Orlando, Florida.
Acknowledgments: Under the direction of the authors, Craig Slawecki, PhD (an employee of CHC), provided writing assistance for this manuscript. Editorial assistance in formatting, proofreading, and copyediting was also provided by CHC. Dr Slawecki has no conflicts of interest to report.
REFERENCES
1. Brown TE. Outside the Box: Rethinking ADD/ADHD in Children and Adults—A Practical Guide. Arlington, VA: American Psychiatric Association Publishing; 2017.
2. Barkley RA. Implications for assessment and clinical management of deficits in executive function. In: Executive Functions: What They Are, How They Work, and Why They Evolved. New York, NY: The Guilford Press; 2012:190-209.
3. Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121(1):65-94. PubMed CrossRef
4. Brown TE. Attention-Deficit Disorders and Comorbidities in Children, Adolescents, and Adults. Arlington, VA: American Psychiatric Press, Inc; 2000.
5. Gioia GA, Isquith PK, Guy SC, et al. BRIEF: Behavior Rating Inventory of Executive Function—Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 2000.
6. Vohs KD, Baumeister RF. Understanding self-regulation: an introduction. In: Vohs KD, Baumeister RF, eds. Handbook of Self-Regulation: Research, Theory, and Applications. New York, NY: Guilford Press; 2004:1-9.
7. Biederman J, Petty C, Fried R, et al. Impact of psychometrically defined deficits of executive functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(10):1730-1738. PubMed CrossRef
8. Brown TE, Reichel PC, Quinlan DM. Executive function impairments in high IQ adults with ADHD. J Atten Disord. 2009;13(2):161-167. PubMed CrossRef
9. Barkley RA, Murphy KR, Fischer M. ADHD in Adults: What the Science Says. New York, NY: The Guilford Press; 2008.
10.Biederman J, Petty CR, Fried R, et al. Discordance between psychometric testing and questionnaire-based definitions of executive function deficits in individuals with ADHD. J Atten Disord. 2008;12(1):92-102. PubMed CrossRef
11.Faraone SV, Biederman J, Spencer T. Diagnostic efficiency of symptom items for identifying adult ADHD. J ADHD Relat Disord. 2010;1:38-48.
12.Kessler RC, Green JG, Adler LA, et al. Structure and diagnosis of adult attention-deficit/hyperactivity disorder: analysis of expanded symptom criteria from the Adult ADHD Clinical Diagnostic Scale. Arch Gen Psychiatry. 2010;67(11):1168-1178. PubMed CrossRef
13.Barkley RA, Murphy KR. Impairment in occupational functioning and adult ADHD: the predictive utility of executive function (EF) ratings versus EF tests. Arch Clin Neuropsychol. 2010;25(3):157-173. PubMed CrossRef
14.Ustun B, Adler LA, Rudin C, et al. The World Health Organization Adult Attention-Deficit/Hyperactivity Disorder Self-Report Screening Scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527. PubMed CrossRef
15.Silverstein MJ, Faraone SV, Leon TL, et al. The relationship between executive function deficits and DSM-5-defined ADHD symptoms. J Atten Disord. 2020;24(1):41-51. PubMed CrossRef
16.Barkley RA. Differential diagnosis of adults with ADHD: the role of executive function and self-regulation. J Clin Psychiatry. 2010;71(7):e17. PubMed CrossRef
17.Barkley RA. ADHD and the Nature of Self Control. New York, NY: Guilford Press; 1997.
18.Barkley RA, Fischer M. Predicting impairment in major life activities and occupational functioning in hyperactive children as adults: self-reported executive function (EF) deficits versus EF tests. Dev Neuropsychol. 2011;36(2):137-161. PubMed CrossRef
19.Brown TE, Flood E, Sarocco P, et al. Persisting psychosocial impairments in adults being treated with medication for attention deficit/hyperactivity disorder [poster]. Presented at American Psychiatric Association; May 20-24, 2017; San Diego, CA.
20.Brown TE. Executive functions and attention deficit hyperactivity disorder: implications of two conflicting views. Int J Disabil Dev Educ. 2006;53(1):35-46. CrossRef
21.Lawson RA, Papadakis AA, Higginson CI, et al. Everyday executive function impairments predict comorbid psychopathology in autism spectrum and attention deficit hyperactivity disorders. Neuropsychology. 2015;29(3):445-453. PubMed CrossRef
22.Dempsey A, Dyehouse J, Schafer J. The relationship between executive function, AD/HD, overeating, and obesity. West J Nurs Res. 2011;33(5):609-629. PubMed CrossRef
23.Thorell LB, Rydell AM, Bohlin G. Parent-child attachment and executive functioning in relation to ADHD symptoms in middle childhood. Attach Hum Dev. 2012;14(5):517-532. PubMed CrossRef
24.Brocki KC, Eninger L, Thorell LB, et al. Interrelations between executive function and symptoms of hyperactivity/impulsivity and inattention in preschoolers: a two year longitudinal study. J Abnorm Child Psychol. 2010;38(2):163-171. PubMed CrossRef
25.Thorell LB. Do delay aversion and executive function deficits make distinct contributions to the functional impact of ADHD symptoms? a study of early academic skill deficits. J Child Psychol Psychiatry. 2007;48(11):1061-1070. PubMed CrossRef
26.Tseng WL, Gau SS. Executive function as a mediator in the link between attention-deficit/hyperactivity disorder and social problems. J Child Psychol Psychiatry. 2013;54(9):996-1004. PubMed CrossRef
27.Sjöwall D, Thorell LB. Functional impairments in attention deficit hyperactivity disorder: the mediating role of neuropsychological functioning. Dev Neuropsychol. 2014;39(3):187-204. PubMed CrossRef
28.Adler LA, Dirks B, Deas PF, et al. Lisdexamfetamine dimesylate in adults with attention-deficit/ hyperactivity disorder who report clinically significant impairment in executive function: results from a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(7):694-702. PubMed CrossRef
29.Craig F, Margari F, Legrottaglie AR, et al. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2016;12:1191-1202. PubMed
30.Roth RM, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) Professional Manual. Lutz, FL: Psychological Assessment Resources; 2005.
31.DuPaul GJ, Power TJ, Anastopoulos AD, et al. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: Guilford Press; 1998.
32.Murphy KR, Adler LA. Assessing attention-deficit/hyperactivity disorder in adults: focus on rating scales. J Clin Psychiatry. 2004;65(suppl 3):12-17. PubMed
33.Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27(2):187-201. PubMed CrossRef
34.Spencer TJ, Adler LA, Weisler RH, et al. Triple-bead mixed amphetamine salts (SPD465), a novel, enhanced extended-release amphetamine formulation for the treatment of adults with ADHD: a randomized, double-blind, multicenter, placebo-controlled study. J Clin Psychiatry. 2008;69(9):1437-1448. PubMed CrossRef
35.Katic A, Dirks B, Babcock T, et al. Treatment outcomes with lisdexamfetamine dimesylate in children who have attention-deficit/hyperactivity disorder with emotional control impairments. J Child Adolesc Psychopharmacol. 2013;23(6):386-393. PubMed CrossRef
36.Adler LA, Alperin S, Leon T, et al. Clinical effects of lisdexamfetamine and mixed amphetamine salts immediate release in adult ADHD: results of a crossover design clinical trial. Postgrad Med. 2014;126(5):17-24. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top