Objective: Serotonin syndrome (SS) is an adverse drug reaction occurring among patients receiving serotonergic agents (SAs), and although SAs are commonly prescribed, the epidemiology and economic burden of SS with concomitant SA use have not been comprehensively examined. The objective of this study was to investigate the prevalence, incidence, and economic burden of SS with SA use.
Methods: A retrospective cohort study was conducted using Veterans Health Administration (VHA) records (identification period: October 1, 2008-September 30, 2012) and commercially insured patient records (Intercontinental Marketing Services PharMetrics Plus; identification period: January 1, 2010-December 31, 2013). Cohorts were based on drug classification and exposure: single monoamine oxidase inhibitor (MAOI), MAOIs in combination with SAs, single non-MAOI SA, and multiple non-MAOI SAs (2, 3, 4, ≥ 5). Participants were aged ≥ 18 years with continuous health plan enrollment for 12 months prior to the first SA claim. Outcomes were SS events (ICD-9-CM: 333.99), annual incidence and prevalence, related health care utilization and costs, and SS incidence relative risk.
Results: Over 15 million patients were identified and categorized by SA prescription type. SS incidence in both populations decreased: 0.19%-0.07% (VHA) and 0.17%-0.09% (commercially insured). Overall SS prevalence decreased during the study period. Compared to single non-MAOI SA patients, SS incidence relative risk was highest among patients prescribed ≥ 5 non-MAOI SAs. Inpatient stays accounted for 4.35% (VHA) and 0.88% (commercially insured) of all SS events. Of SS-related inpatient stays, median costs were $8,765 (VHA) and $10,792 (commercially insured).
Conclusions: SS incidence and prevalence and SS-related hospitalization risk among patients prescribed SAs were low in both populations. This study provides additional information regarding SS risk associated with SA use.
Objective: Serotonin syndrome (SS) is an adverse drug reaction occurring among patients receiving serotonergic agents (SAs), and although SAs are commonly prescribed, the epidemiology and economic burden of SS with concomitant SA use have not been comprehensively examined. The objective of this study was to investigate the prevalence, incidence, and economic burden of SS with SA use.
Methods: A retrospective cohort study was conducted using Veterans Health Administration (VHA) records (identification period: October 1, 2008-September 30, 2012) and commercially insured patient records (Intercontinental Marketing Services PharMetrics Plus; identification period: January 1, 2010-December 31, 2013). Cohorts were based on drug classification and exposure: single monoamine oxidase inhibitor (MAOI), MAOIs in combination with SAs, single non-MAOI SA, and multiple non-MAOI SAs (2, 3, 4, ≥ 5). Participants were aged ≥ 18 years with continuous health plan enrollment for 12 months prior to the first SA claim. Outcomes were SS events (ICD-9-CM: 333.99), annual incidence and prevalence, related health care utilization and costs, and SS incidence relative risk.
Results: Over 15 million patients were identified and categorized by SA prescription type. SS incidence in both populations decreased: 0.19%-0.07% (VHA) and 0.17%-0.09% (commercially insured). Overall SS prevalence decreased during the study period. Compared to single non-MAOI SA patients, SS incidence relative risk was highest among patients prescribed ≥ 5 non-MAOI SAs. Inpatient stays accounted for 4.35% (VHA) and 0.88% (commercially insured) of all SS events. Of SS-related inpatient stays, median costs were $8,765 (VHA) and $10,792 (commercially insured).
Conclusions: SS incidence and prevalence and SS-related hospitalization risk among patients prescribed SAs were low in both populations. This study provides additional information regarding SS risk associated with SA use.
Prim Care Companion CNS Disord 2017;19(6):17m02200
https://doi.org/10.4088/PCC.17m02200
© Copyright 2017 Physicians Postgraduate Press, Inc.
aLong Beach Veterans Affairs Healthcare System, Long Beach, California
bUniversity of California Irvine School of Medicine, Irvine, California
cSTATinMED Research, Ann Arbor, Michigan
dSouthern California Institute of Research and Education, Long Beach, California
eCenter for Innovation & Outcomes Research, Department of Surgery, Columbia University, New York, New York
fMEF University, Istanbul, Turkey
gEisai Inc, Woodcliff Lake, New Jersey
*Corresponding author: Lin Xie, MA, MS, STATinMED Research, 211 N Fourth Ave, Ste 2B, Ann Arbor, MI 48104 ([email protected]).
Serotonin syndrome (SS) is an adverse drug reaction thought to occur as a result of an increase in the postsynaptic concentrations of serotonin.1 Characterized by mental status changes (eg, anxiety, delirium, confusion, drowsiness, seizures, coma), autonomic instability (eg, hyperthermia, sinus tachycardia, blood pressure changes, flushing, diarrhea, vomiting), and neuromuscular hyperactivity (eg, akathisia, myoclonus, tremor, nystagmus, shivering), the symptoms of SS can range from mild to fatal.2-4 Most reported cases of SS are in patients using multiple serotonergic agents (SAs) or those who have had considerable exposure to a single serotonin-augmenting drug.5
Serotonin syndrome has been associated with certain pathophysiologic mechanisms, such as inhibition of serotonin reuptake, as seen in the use of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants. Other mechanisms linked to SS include decrease in the breakdown of serotonin (as observed in monoamine oxidase inhibitors [MAOIs]), increase in serotonin precursors or agonists such as triptans, enhancement of serotonin release (eg, stimulants),6 or interference with the breakdown of SAs.7 Many medications approved by the US Food and Drug Administration (FDA) carry warnings for SS, including commonly prescribed medications such as antidepressants, antipsychotics, antimigraine medications, or medications prescribed for the management of Parkinson’s disease and Alzheimer’s disease.8-10
In July 2006, the FDA issued a warning that the use of a triptan in combination with an SSRI or SNRI may result in a potentially life-threatening problem known as SS.11,12 Clinicians were cautioned to monitor for potential risk of serotonin toxicity.13 However, in 2010, the American Headache Society13 published a position paper stating that the basis of the 2006 FDA warning—a collection of case studies—was conflicting and inadequate to determine the risk of SS with SA use.14 Although the quantity and extent of concomitant use of a triptan and an SSRI or SNRI within the United States can be approximated, the quantity of cases of SS associated with the concomitant use of SAs is still lacking in the literature.15 A significant gap remains in the literature between the actual incidences of SS cases and resulting clinical and economic consequences due to concomitant SA use.15 Without these data, it remains difficult to assess the risk-benefit associated with the use of SAs. A study16 of elderly Australian veterans and dependents showed widespread use of serotonergic medications. Approximately 42% of the study population was prescribed at least 1 SA, 8% was prescribed a combination of SAs, and roughly 0.7% was prescribed MAOIs in combination with SAs. Despite these data, the authors failed to report the actual incidence of SS.16
The goal of this study was to examine the prevalence and incidence of SS over time and the economic impact of SS with concomitant use of SAs in clinical practice in 2 different US populations using large, nationally representative databases. To our knowledge, this is the first large-scale study assessing the prevalence, incidence, and economic impact of SS with concomitant use of SAs.
METHODS
Data Source
This was a retrospective claims data study using medical, pharmacy, and enrollment information to identify epidemiologic patterns and prescribing practices associated with SS using 2 US national datasets: the Veterans Health Administration (VHA) dataset from October 1, 2007-September 30, 2012 and commercially insured patients in the Intercontinental Marketing Services PharMetrics Plus (IMS) dataset from January 1, 2009-December 31, 2013.
The VHA dataset contains data for VHA-provided health care utilized primarily by US military veterans and a small number of nonveterans (eg, employees, eligible family members, research participants). In the VHA dataset, costs for utilization are based on payments from the government.
The commercially insured patient dataset (IMS) contains information for more than 71 million patients using over 103 health plans across the United States. The State Children’s Health Insurance Program, Medicaid, Medicare Advantage, self-insured, and Medicare supplemental health plans are also available in the commercially insured patient dataset. Institutional review board approval was not required for this retrospective study as its undertaking involved no collection or use of personally identifiable information.17 In the commercially insured patient dataset, cost of utilization is based on the amount paid by the health plan.

- Current research supports evidence that, using Veterans Health Administration (VHA) and IMS PharMetrics Plus (IMS) databases across 24 million (VHA) and 40 million (IMS) patients, overall serotonin syndrome (SS) incidence and prevalence were low among patients prescribed serotonergic agents (SAs) in the United States and trending to decline between years studied.
- Severe SS events leading to emergency department visits or hospitalizations were rare with no death reports; in both VHA and general (IMS) populations, the proportion of patients with SS-related hospitalizations increased as the number of non-MAOI SAs increased.
- This study provides practical information that can help health care professionals better understand the benefit and risk of SA use in their prescribing practices.
Patient Identification
SS prevalence and incidence. SS was identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code 333.99. As ICD-10 had not yet been implemented during the study period, ICD-9 was the diagnostic tool used during the study period analyses. Primary and secondary diagnoses were used to calculate incidence and prevalence of SS for the overall population represented by each dataset and among patients prescribed SAs.
Patients included in the SS prevalence analysis had continuous health plan enrollment for the entire fiscal year (VHA: 2008-2012) or calendar year (commercially insured patients: 2009-2013). The prevalence of SS was calculated by dividing the number of patients with at least 1 medical claim for SS by the number of patients in the overall population or those with ≥ 1 pharmacy claim for an SA.
Patients were included in the SS incidence analysis if they had continuous health plan enrollment for the entire fiscal year (VHA: 2009-2012) or calendar year (commercially insured patients: 2010-2013) and the prior year. The incidence of SS was calculated by dividing the number of patients with at least 1 new medical claim for SS by the number of patients in the overall population and in those with ≥ 1 SA prescription.
SA prescribing practices. An analysis of the prescribing patterns of concomitant medications associated with SS (Supplementary Table 1) was also conducted. All patients were required to have an SS diagnosis. Patients were identified from January 1, 2010-December 31, 2013, in the commercially insured patient dataset and from October 1, 2009-September 30, 2012, in the VHA dataset. Patients aged 18 years or older with ≥ 1 prescription claim for SAs and with continuous medical and pharmacy benefits for at least 12 months preindex date were included. The first SA prescription date was designated as the index date, and patient data were observed until either the earliest date of health plan disenrollment, the end of the study period, or death.
Cohort assignment. Patients prescribed at least 1 SA and diagnosed with SS were categorized into cohorts on the basis of their treatment initiation (Supplementary Figure 1). If there were multiple prescriptions, an overlap period of at least 1 day for each drug was required for all medication combinations. Combinations were defined as a pharmacy claim for 1 drug on or before the run-out date (prescription date + number of days’ supply) of the other drug. New pharmacy prescriptions were checked as were the number of drugs in supply at the time of the new prescription. A new prescription with or without an overlap period with another previous prescription was considered the start of a new observation period.
Based on their relevant medication exposure during the follow-up period, patients were assigned to 1 of 7 cohorts. Cohorts were assigned and categorized by number and type of medication. The 1-drug cohort included patients prescribed 1 SA, excluding MAOIs. The 1 MAOI drug cohort included those who were prescribed 1 MAOI agent. The MAOI combination cohort described those prescribed a combination of MAOIs or any other SAs. The remaining cohorts were enumerated according to the number of SAs prescribed, excluding MAOIs: the 2-drug combination cohort included patients prescribed a combination of 2 SAs, except MAOIs; the 3-drug combination cohort, a combination of 3 SAs, except MAOIs; the 4-drug combination cohort, a combination of 4 SAs, except MAOIs; and the ≥ 5-drug combination cohort, a combination of ≥ 5 SAs, except MAOIs.
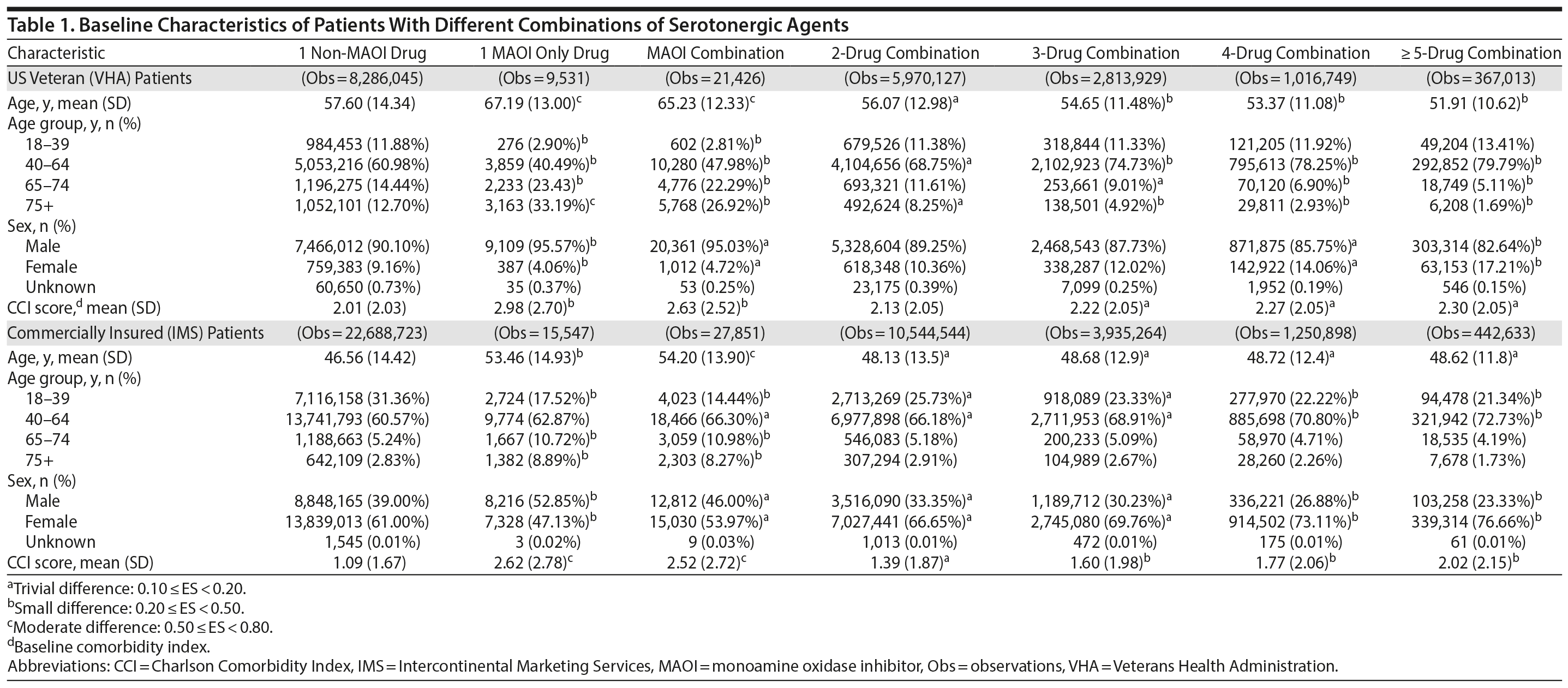
Baseline variables. Baseline demographic variables included age, sex, and US region (Northeast, North Central, South, and West). The Charlson Comorbidity Index (CCI)18-20 score, a weighted summation of 23 comorbid conditions and warfarin use, was also calculated. Some SAs in combination with warfarin are known to increase the risk of bleeding in some patients.21
Outcome variables. All outcomes were evaluated for both populations overall by dataset. The prevalence and incidence of SS were calculated for the overall population and for patients with ≥ 1 pharmacy claim for an SA and were reported as a percentage. The SS incidence rate was calculated as the incidence rate (per 1,000 person-years) using the number of new SS cases divided by the total person-years at risk.
Health care costs and utilization per SS event were calculated and defined as the health care costs and utilization associated with a primary SS diagnosis. Health care utilization per SS event was estimated, and the SS-associated visits were reported: inpatient stays, emergency department (ED) visits, office and outpatient visits, and length of stay. One event was considered as ≥ 1 SS diagnosis claim occurring within ± 1 day. SS-related health care costs were also estimated, including inpatient, ED, office, outpatient, and total medical (inpatient + outpatient) costs.
Data analysis. Seven cohorts were compared based on drug exposure: 1 non-MAOI drug, 1 MAOI drug, MAOI combination, 2-drug combination, 3-drug combination, 4-drug combination, and ≥ 5-drug combination cohorts. Descriptive analyses were performed for comparisons of all baseline and outcome variables. Percentages and counts were provided for dichotomous and polychotomous variables. Means and standard deviations (SDs) were calculated for continuous variables. Student t tests were used to examine differences in continuous variables of interest between study cohorts. Chi-square tests of proportion were used to examine bivariate associations for categorical variables. Effect size (ES)—defined as the absolute difference in sample means divided by an estimate of the pooled ES of each variable—was provided. The ES helps to distinguish practical (ie, clinical) versus statistical significance in situations in which there are very large differences in sample size between groups as seen in this study. The ES was reported as 100 times the absolute value of the actual ES to allow for an easy interpretation. Any ES > 10 was considered significant.22
Poisson regression was used to evaluate SS incidence trends among the cohorts. Adjusted incidence relative risk (IRR) for SS was estimated using the 1 non-MAOI drug cohort as the reference group, and corresponding P values and 95% confidence intervals (CIs) were calculated. All statistical analyses were conducted in SAS version 9.3.
RESULTS
A total of 3,349,984 US veterans and 11,818,956 commercially insured patients were identified for this study.
SS Incidence and Prevalence Trends
Figure 1 shows the SS incidence and prevalence trends in the overall population and in patients prescribed SAs during the study period.
In the VHA population, a decrease occurred in the SS prevalence rate from 2009 to 2012 for the overall population (0.06%-0.02%) and for patients prescribed SAs (0.19%-0.07%). The incidence of SS also decreased from 2008 to 2012 in the overall population (0.13%-0.07%) and in patients prescribed SAs (0.47%-0.23%).
Similarly, in the commercially insured population, a decrease occurred in the SS incidence rate from 2010 to 2013 for the overall population (0.06%-0.03%) and for patients prescribed SAs (0.17%-0.09%). A decrease in the SS prevalence rate was also observed from 2009 to 2013 in the overall population (0.10%-0.05%) and in patients prescribed SAs (0.28%-0.14%).
Patient Characteristics
Patient characteristics are summarized in Table 1. Compared to the commercially insured patients, US veterans were mostly male and had higher mean CCI scores, indicating a less healthy population. Overall, US veterans were older than commercially insured patients. In the VHA population, those patients who were prescribed 1 MAOI SA were also the oldest (aged 67 years). Likewise, the commercially insured patients prescribed MAOI drug combinations were the oldest in that population (aged 54 years). In both populations, patients prescribed 1 non-MAOI SA had the lowest CCI scores (Table 1).

SS Incidence Rate
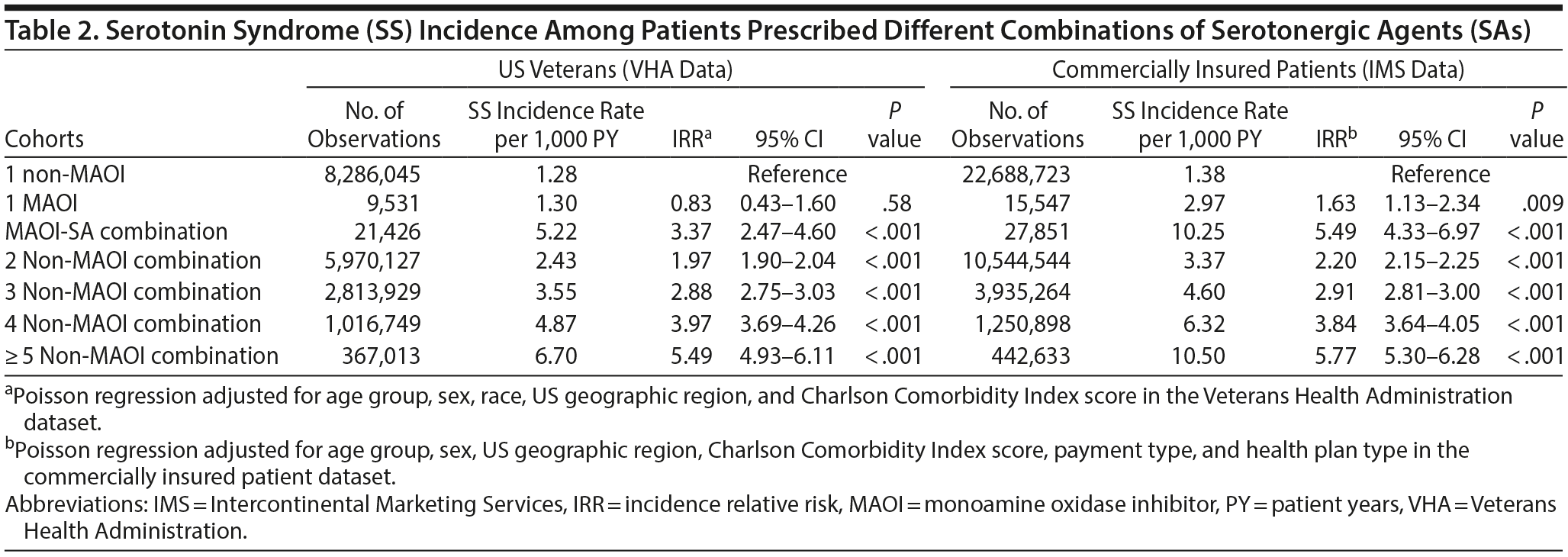
The SS incidence rate per 1,000 person-years and the IRR for the 7 cohorts are shown in Table 2. The highest incidence rate (per 1,000 person-years) was observed in patients who were prescribed MAOIs in combination with SAs (5.22, 5.49) and patients with ≥ 5 non-MAOI SAs (6.70, 10.50) for VHA and commercially insured patients, respectively. The lowest SS incidence rate was seen among those prescribed a single non-MAOI SA. Furthermore, a corresponding increase occurred in the SS incidence rate as the number of drugs in the combination increased in the absence of MAOIs. Within the VHA population, patients prescribed ≥ 5 non-MAOI SAs had the highest adjusted IRR (IRR = 5.49; 95% CI, 4.93-6.11). Analysis of the commercially insured patient dataset revealed the same trend as the VHA dataset.
Health Care Resource Utilization and Cost Per SS Event
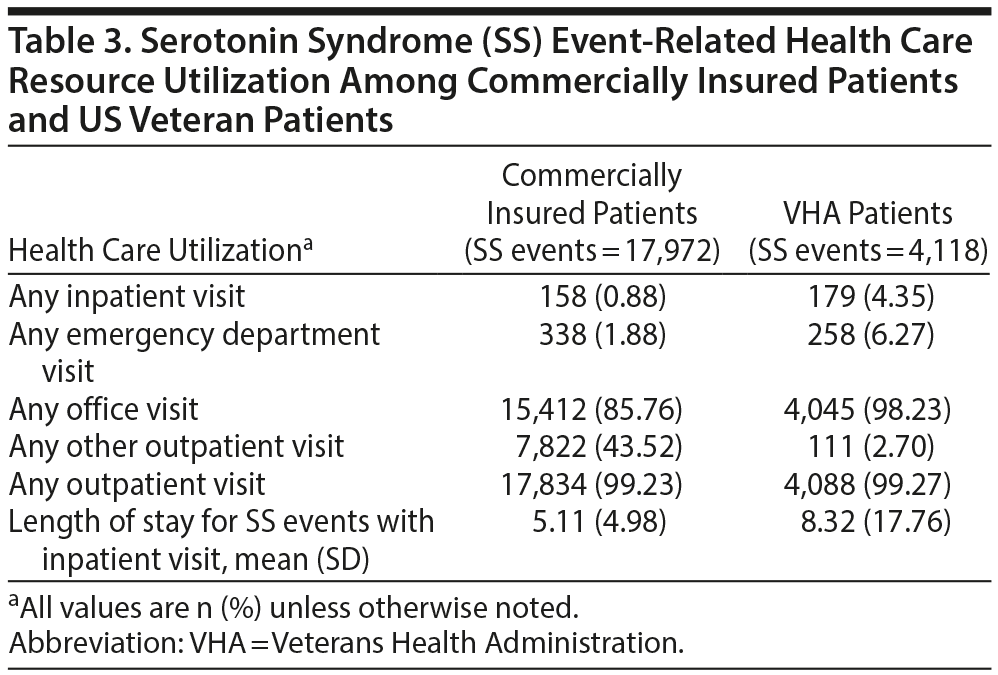
Claims with SS as the primary diagnosis were used to calculate health care resource utilization and costs per SS event. Table 3 displays the health care resource utilization per SS event for both patient populations. Within the veteran population, 4.35% of all SS events led to a hospitalization, and 6.27% led to an ED visit. The mean length of stay was 8.32 days for SS hospitalization (Table 3). For the commercially insured patient population, 0.88% of all SS events led to a hospitalization and 1.88% led to an ED visit. The mean length of stay was 5.11 days for SS involving an inpatient stay (Table 3). In both populations, the proportion of patients with SS-related hospitalizations increased as the number of non-MAOI SAs increased.
Figure 2 shows the health care costs per SS event for both patient populations. In the VHA population, the mean total health care costs per SS event were the highest for patients in the ≥ 5 non-MAOI drug combination cohort ($3,837). Approximately 84% of the mean total health care costs for patients prescribed ≥ 5 non-MAOI drugs in combination were due to inpatient stay costs. The mean total health care costs per SS event within the MAOI combination cohort were $2,896 (Figure 2). The median costs per SS-related inpatient stay, regardless of SA category, were $8,765 (Supplementary Table 2).
For commercially insured patients, total health care costs per SS event were, on average, the highest for patients in the MAOI combination cohort at $2,474. Similarly, the main cost driver was inpatient care costs. For the ≥ 5 non-MAOI drug combination cohort, the mean total health care costs per SS event were $1,167. The median cost per SS-related inpatient stay was $10,792 (Supplementary Table 2).
Overall, the mean total health care costs were higher in the VHA population compared to the commercially insured population (Figure 2). No deaths were observed in association with SS events in either patient population.
DISCUSSION
The goal of this study was to establish the epidemiologic and prescription patterns along with the economic impact of SS, using large-scale, diverse datasets from VHA and commercially insured patient data with potentially generalizable sampling. We believe this goal is meaningful and pragmatic in light of established multiple SA prescribing practices across clinical settings.
In contrast to previous studies, which focused on SS incidence analysis relevant to a specific medication, our study encapsulated the overall incidence and prevalence of SS by examining several medications.13,23 Patient data samples used for this study were vastly different from each other. The commercially insured patients had lower mean CCI scores, indicating a healthier population compared to the VHA population. Commercially insured patients had a higher proportion of females compared to the VHA population. The study results revealed corresponding trends in incidence, prevalence, and economic burden among the 2 populations, but the numbers were different, particularly for the ED visits and SS event-related hospitalizations, which were higher within the VHA population.
As expected, the incidence and prevalence of SS were low among patients prescribed SAs. Over the study period, the incidence and prevalence of SS in both populations using SAs consistently decreased. This finding is perplexing given that the total number of SAs prescribed (antidepressants included) has increased over time.24 A possible explanation is that the SS warning issued by the FDA in 200612 may have led to an initial increase in the incidence of SS due to heightened awareness and possible overdiagnosis of SS by physicians who prescribed SAs. After the SS warnings were removed from electronic health records,11 SS incidence decreased gradually, as indicated by the current study. Another hypothetical explanation of this decrease is that it is a result of more physicians with a better understanding of SS and its association with concomitant SAs. Following this reasoning, physicians may be taking preventative measures before prescribing SAs to vulnerable patients.
Furthermore, the IRR associated with the use of SAs, such as fluoxetine and paroxetine, was examined. Fluoxetine and paroxetine inhibit the cytochrome P450 2D6 enzyme that metabolizes other SAs. This inhibition leads to increased levels of SAs in the body and theoretically may eventually lead to an increased risk of SS.25,26 Despite a heightened risk of SS associated with use of either fluoxetine or paroxetine in combination with other SAs, our study found that the ≥ 5 non-MAOI drug combination and MAOI combination cohort had the highest IRR associated with SS.
After adjusting for demographic and clinical characteristics, patients prescribed multiple drugs in combination were more likely to be diagnosed with SS compared to those prescribed 1 SA. Moreover, a direct increase in IRR with an increase in SA use was noted. This correlational relationship may have occurred as a result of the dose-response effect of the SAs in the body, increasing the risk of serotonin toxicity.13
The use of nondisclosed over-the-counter medication such as St John’s Wort, a complementary and alternative medicine used to treat depression, has been known to interact with some SAs, resulting in SS.27 In other cases, SS may result from noncompliance with medication instructions or unintentional overdose of serotonergic medication.28 SA prescriptions filled over-the-counter or provided as samples by physicians were not included in the datasets. In addition, the presence of a claim for a filled prescription did not signify whether the medication was taken as prescribed.
Another facet of our study examined and described health care costs and utilization related to SS events among the US veteran and commercially insured patients. To our knowledge, this is the first study to assess the economic burden and health resource utilization of SS using real-world data. In this analysis, we observed fewer cases of severe SS events as indicated by a very low percentage of ED visits and hospitalizations due to an SS event. The median inpatient cost for SS was highest for those prescribed more than 5 combinations of non-MAOI SAs or an MAOI combination compared to those prescribed 1 non-MAOI SA. We hypothesize this difference to be the result of a higher level of serotonin within these patients, leading to more severe overall clinical presentations of SS and, ultimately, a higher economic burden.
Limitations
Claims data are valuable for the efficient and effective examination of real-world health information but possess inherent limitations. The ICD-9 code used to identify SS is also used for other conditions, such as drug-induced akathisia; therefore, the study results for prevalence and incidence of SS may have been overestimated.29 A diagnosis code on a medical claim is not indicative of a positive disease presence as it may have been incorrectly coded or included as rule-out criteria rather than actual disease. Data entry errors also may have occurred. Patients’ use of multiple health systems could potentially lead in some cases to misclassification of SS patients prescribed SAs.
In conclusion, SS incidence and prevalence were low among patients prescribed SAs in the US veteran and commercially insured populations. Severe SS events leading to emergency room visits or hospitalizations are rare. However, study findings suggest that relative risk of SS or SS-related hospitalization increases as the number of concomitant SAs increases.
CONCLUSION
This study provides practical information and may be useful to practitioners in the revision of current and future prescribing practices. Future research is needed to better understand potential predisposing factors leading to SS.
Submitted: July 25, 2017; accepted October 11, 2017.
Published online: December 28, 2017.
Author contributions: Ms Xie and Dr Baser, of STATinMED Research, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The study hypothesis was developed by Dr Nguyen, who asked for technical support to answer these questions. Drs Nguyen and Wang and Ms Alley conceptualized and designed the study. Dr Baser and Ms Xie collected and analyzed the data. Drs Nguyen, Baser, and Wang and Mss Xie and Alley substantially contributed to the interpretation of the data. Drs Nguyen and Baser and Mss Alley and Xie wrote the manuscript and/or substantially contributed to critical revisions of the intellectual content. All authors agree to the final version.
Potential conflicts of interest: Dr Nguyen owns stock in Orexigen; is a consultant and member of the speakers’ bureau to Otsuka; received a research grant from Forest Laboratories; and was a consultant and member of the speakers’ bureau to Eisai at the time of study inception and execution; however, he received no travel or research funding for this study. Ms Alley is an employee of the Veterans Affairs Long Beach Healthcare System and a paid consultant to the Southern California Institute of Research and Education and Eisai. Dr Baser and Ms Xie are employees of STATinMED Research, which is a paid consultant to Eisai in connection with the study design, data analysis, and development of this manuscript. Dr Wang is an employee of Eisai. Dr McCarron has no conflicts of interest related to the subject of this article.
Funding/support: This study was funded by Eisai Inc.
Role of sponsor: Eisai Inc supplied support and participated in formulating the outline of the study but had no role in the selection or interpretation of evidence. Although Eisai staff reviewed the manuscript, final approval for the decision to submit the manuscript was the sole decision of the authors.
Previous presentations: The results of this study were previously presented as poster presentations at the following events: American Society of Clinical Psychopharmacology Annual Meeting; June 22-25, 2015; Miami, Florida ▪ American Association of Nurse Practitioner National Conference; June 9-14, 2015; New Orleans, Louisiana ▪ International Society for Pharmacoeconomics and Outcomes Research 20th Annual International Meeting; May 16-20, 2015; Philadelphia Pennsylvania ▪ American Psychiatric Association 168th Annual Meeting; May 16-20, 2015; Toronto, Ontario, Canada ▪ Society of General Internal Medicine 38th Annual Meeting; April 22-25, 2015; Toronto, Ontario, Canada.
Acknowledgments: Statistical analysis support was provided by Furaha Kariburyo, MPH, and Yuexi Wang, MS, of STATinMED Research. Medical writing support was provided by Adesuwa Ogbomo, MPH, of STATinMED Research. Editing support was provided by Michael Moriarty, BS, of STATinMED Research. All acknowledged contributors are members of STATinMED Research, which is a paid consultant to Eisai Inc.
Additional information: The data utilized for the study described herein are not available to the public.
Supplementary material: See accompanying pages.
REFERENCES
1. Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol. 1999;13(1):100-109. PubMed CrossRef
2. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642. PubMed CrossRef
3. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619. PubMed CrossRef
4. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120. PubMed CrossRef
5. Sclar DA, Robison LM, Skaer TL. Concomitant triptan and SSRI or SNRI use: a risk for serotonin syndrome. Headache. 2008;48(1):126-129. PubMed
6. Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318. PubMed
7. Talarico G, Tosto G, Pietracupa S, et al. Serotonin toxicity: a short review of the literature and two case reports involving citalopram. Neurol Sci. 2011;32(3):507-509. PubMed CrossRef
8. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913. PubMed CrossRef
9. Loder E. Triptan therapy in migraine. N Engl J Med. 2010;363(1):63-70. PubMed CrossRef
10. Chiuccariello L, Cooke RG, Miler L, et al. Monoamine oxidase—a occupancy by moclobemide and phenelzine: implications for the development of monoamine oxidase inhibitors. Int J Neuropsychopharmacol. 2015;19(1). PubMed CrossRef
11. Schuman E. Is an FDA alert harming patients? JAAPA. 2009;22(5):55. PubMed
12. Combined Use of 5-Hydroxytryptamine Receptor Agonists (Triptans), Selective Serotonin Reuptake Inhibitors (SSRIs) or Selective Serotonin/Norepinephrine Reuptake Inhibitors (SNRIs) May Result in Life-Threatening Serotonin Syndrome. https://wayback.archive-it.org/7993/20170406044820/
https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/
ucm124349.htm. Accessed December 12, 2017.
13. Evans RW, Tepper SJ, Shapiro RE, et al. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099. PubMed CrossRef
14. Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52(2):198-203. PubMed CrossRef
15. Evans CE, Sebastian J. Serotonin syndrome. Emerg Med J. 2007;24(4):e20. PubMed CrossRef
16. Ringland C, Mant A, McGettigan P, et al. Uncovering the potential risk of serotonin toxicity in Australian veterans using pharmaceutical claims data. Br J Clin Pharmacol. 2008;66(5):682-688. PubMed
17. US Department of Health and Human Services. Health Insurance Portability and Accountability Act 1996. Public law 104-191, 104th Congress. https://www.gpo.gov/fdsys/pkg/PLAW-104publ191/pdf/PLAW-104publ191.pdf. Accessed December 12, 2017.
18. Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251. PubMed CrossRef
19. de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity: a critical review of available methods. J Clin Epidemiol. 2003;56(3):221-229. PubMed CrossRef
20. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. PubMed CrossRef
21. Quinn GR, Singer DE, Chang Y, et al. Effect of selective serotonin reuptake inhibitors on bleeding risk in patients with atrial fibrillation taking warfarin. Am J Cardiol. 2014;114(4):583-586. PubMed CrossRef
22. Cohen J. A power primer. Psychol Bull. 1992;112(1):155-159. PubMed CrossRef
23. Koury KM, Tsui B, Gulur P. Incidence of serotonin syndrome in patients treated with fentanyl on serotonergic agents. Pain Physician. 2015;18(1):E27-E30. PubMed
24. Kantor ED, Rehm CD, Haas JS, et al. Trends in Prescription Drug Use Among Adults in the United States From 1999-2012. JAMA. 2015;314(17):1818-1831. PubMed CrossRef
25. Nemeroff CB, DeVane CL, Pollock BG. Newer antidepressants and the cytochrome P450 system. Am J Psychiatry. 1996;153(3):311-320. PubMed CrossRef
26. Wigen CL, Goetz MB. Serotonin syndrome and linezolid. Clin Infect Dis. 2002;34(12):1651-1652. PubMed CrossRef
27. Davis SA, Feldman SR, Taylor SL. Use of St. John’s Wort in potentially dangerous combinations. J Altern Complement Med. 2014;20(7):578-579. PubMed CrossRef
28. Martini DI, Nacca N, Haswell D, et al. Serotonin syndrome following metaxalone overdose and therapeutic use of a selective serotonin reuptake inhibitor. Clin Toxicol (Phila). 2015;53(3):185-187. PubMed CrossRef
29. ICD-9data.com website. http://www.icd9data.com/2015/Volume1/320-389/330-337/333/333.99.htm. Accessed September 8, 2015.
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top