ABSTRACT
Objective: To assess the role of high-sensitivity C-reactive protein (hs-CRP) as a biomarker, trait marker, and endophenotype in mania.
Methods: Forty patients with mania, 40 of their first-degree relatives, and 30 healthy controls were recruited via a purposive sampling method from May 2020 to February 2021. hs-CRP levels were measured in all groups at baseline. The patient group was evaluated with the Young Mania Rating Scale, and hs-CRP levels were assessed in all participants at baseline, 2 weeks, and 6 weeks. Data were analyzed with SPSS version 25.
Results: hs-CRP levels were significantly higher in patients than in controls and first-degree relatives (P = .001). However, hs-CRP levels were not higher in first-degree relatives compared to healthy controls. There was a significant reduction in total YMRS and domain scores and hs-CRP levels in patients at weeks 2 and 6 compared to baseline (P = .02).
Conclusions: The blood hs-CRP level is a biomarker in mania, which may be a newer approach to detect disease progression and perhaps guide novel therapies. hs-CRP as an endophenotype requires further evaluation in future studies.
Prim Care Companion CNS Disord 2022;24(6):21m03194
To cite: Abshad M, Das B, Kumar M, et al. Role of high-sensitivity C-reactive protein as a biomarker and endophenotype in mania. Prim Care Companion CNS Disord. 2022;24(6):21m03194.
To share: https://doi.org/10.4088/PCC.21m03194
© 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Central Institute of Psychiatry, Ranchi, India
bDr Baba Sahib Ambedkar Medical College and Hospital, New Delhi, India
cAll India Institute of Medical Sciences, Guwahati, India
dDepartment of Biochemistry, Central Institute of Psychiatry, Ranchi, India
*Corresponding author: Pranjal Dey, MD, All India Institute of Medical Sciences, Silbharal Changsari, Guwahati, Assam 781101, India ([email protected]).
Mood disorders are increasingly recognized as having a strong association with a proinflammatory state, providing the basis for investigating novel therapeutic targets.1 The term biomarker, a portmanteau of biological marker, refers to “any substance, structure, or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease.”2 The cytokines and chemical factors produced during inflammatory responses serve as excellent biomarkers when investigating the potential relationship between inflammation and mood disorders.3,4 Proinflammatory markers have shown substantial evidence for increased inflammation during manic and depressive episodes, with particularly increased levels of C-reactive protein (CRP), cytokines, and tumor necrosis factor α (TNF-α).5 Despite a growing body of evidence regarding the involvement of cytokines in psychiatric and neurodegenerative disorders, findings are still limited in mood disorders.
CRP and hs-CRP as Inflammatory Markers in Mood Disorders
Inflammation is a condition characterized by cytokine cascades, cellular immune responses with increased levels of acute phase proteins, and complement factors. Interleukin-1 (IL-1) and TNF are the primary inflammatory mediators that induce the production of acute phase proteins, such as haptoglobin and CRP.6 CRP is a pentameric protein that is generated in the liver and secreted in the blood, and its measurement provides a reliable marker of chronic inflammation caused by infectious and other inflammatory agents.7 Traditional CRP measurement only detects CRP in the range of 10 to 1,000 mg/L, whereas high-sensitivity CRP (hs-CRP) detects levels in the range of 0.5 mg/L to 10 mg/L. In simpler terms, hs-CRP measures trace amounts of CRP in the blood.8 A few studies have measured the prevalence of hs-CRP, a more sensitive assay, in bipolar disorder patients. Two studies9,10 showed that patients with manic episodes had higher mean hs-CRP levels than healthy controls. Another study11 found that the levels of hs-CRP were increased in manic patients compared to euthymic and depressed patients, suggesting that the episodes of mania are particularly sensitive to inflammatory changes.
Role of CRP and hs-CRP as Trait Markers in Mood Disorders
The concept of the endophenotype was introduced to psychiatry over 30 years ago but has gained popularity more recently.12 In psychiatry research, a biomarker may be called an endophenotype when it is heritable and segregate with illness in the population. It must co-segregate with illness within families and not be state dependent. Also, it must be amenable to reliable measurement, be specific to the illness of interest, and present at a higher rate within affected families than in the population.13 Thus, endophenotypes may be useful for many reasons, such as indicating trait markers of susceptibility to psychiatric illness by providing biological markers of the disease. Studies7,11,14 established that a higher level of hs-CRP as a biological marker in manic episodes of bipolar disorder was correlated with Young Mania Rating Scale (YMRS)15 scores for evaluating treatment response. Thus, hs-CRP was interpreted as an episode-specific marker. The higher hs-CRP levels observed in patient groups compared to controls before and after response to treatment in those studies7,11,14 suggest that hs-CRP may be a trait marker in bipolar disorder.
Thus, hs-CRP is a potential marker of bipolar disorder; however, the nature of the association is uncertain. The objective of this study was to investigate hs-CRP as a biomarker, trait marker, and endophenotype in mania.
METHODS
Sample
This was a hospital-based prospective study. The sample was recruited via a purposive sampling method from May 2020 to February 2021. The sample included inpatients from the department of psychiatry of a tertiary care hospital located in eastern India, their first-degree relatives, and healthy individuals as controls.
Patients
After informed consent was obtained, 40 patients aged 18–60 years diagnosed with first-episode mania or bipolar affective disorder, current episode mania as per ICD-10 criteria with a YMRS score > 20 were included in the study.16 We included only those aged ≥ 18 years because findings based on studies of childhood-onset bipolar disorder may not be applicable to adult-onset bipolar disorder and vice versa.17 Patients with present or prior history of any major physical or psychiatric illness other than manic episode were excluded. Patients with any other comorbid substance dependence except nicotine and caffeine, those with any infectious disease during the study, and pregnant women and lactating mothers were excluded. These patients were excluded because all these conditions are known to alter the phenomenology and course of the disorder as well as levels of the biomarkers.5 Patients with previous episodes of either unipolar or bipolar depression were excluded.18,19
First-Degree Relatives
This group comprised 40 first-degree relatives of the selected patients (ie, twin sibling > sibling [male > female] > parents [father > mother]) who were willing to provide informed consent. Individuals with present or prior history of any major physical or psychiatric illness and having any other substance dependence except nicotine and caffeine were excluded. Pregnant women, lactating mothers, and individuals with any infectious disease during the study were excluded.5
Controls
The control group comprised 30 individuals who worked at the hospital and had no psychiatric or major medical illness. They were required to have a 12-item General Health Questionnaire (GHQ-12)20 score < 3 and to provide informed consent. Those with a family history of mood disorder, substance dependence except for nicotine or caffeine, or infectious disease during the study, as well as pregnant women and lactating mothers were excluded.5 The controls were demographically matched with the patients.
Instruments
Sociodemographic and clinical data sheet. A semistructured proforma for recording demographic details such as age, sex, marital status, religion, education, occupation, income, and socioeconomic status was created. Also, clinical data were recorded by interviewing the patient and from the case record file. The form also included history of medical and psychiatric illness, treatment history, and details of the physical and mental status examination. Finally, diagnosis of the patient was made according to ICD-10 diagnostic criteria for research.16
YMRS. The YMRS is a rating scale used to evaluate manic symptoms at baseline and over time in individuals with mania. It has 11 items and is based on the patient’s subjective report of his/her clinical condition over the previous 48 hours. The YMRS score was > 20 for the patient group at baseline.15
GHQ-12. The GHQ-12 is a screening instrument used to identify minor psychiatric disorders in the general population and within community or nonpsychiatric clinical settings such as primary care or in general medical outpatients.20
hs-CRP by Immunoturbidimetric Assay
Blood was drawn from patients, first-degree relatives, and healthy controls and allowed to clot at room temperature in a plain vial. It was centrifuged at approximately 2,500 rpm for 5 minutes. The serum was analyzed for levels of hs-CRP using immunoturbidimetric assay, which is based on the principle of agglutination reaction. The test specimen was mixed with activation buffer and latex reagent and allowed to react. The presence of CRP in the test specimen resulted in the formation of an insoluble complex producing turbidity, which was measured at a wavelength of 630 nm. The increase in turbidity corresponded to the concentration of CRP in test specimens measured at baseline, 2 weeks, and 6 weeks.
Operational Procedure
The study was initiated after obtaining approval from the hospital’s institutional ethics committee. Clinically diagnosed patients with first-episode mania or bipolar affective disorder, current episode mania who fulfilled the inclusion and exclusion criteria were included in the study. The YMRS was administered to the patient group to measure the total score and different parameters of mania. Sociodemographic and clinical information was obtained from patients, their first-degree relatives, and controls. Infections were ruled out in all the 3 groups by detailed clinical examination and via blood examination in parameters of total leukocyte count, differential leukocyte count, and erythrocyte sedimentation rate, which had to be within normal range.21 Patients were started on mood stabilizers, antipsychotics, or benzodiazepines as decided by the treatment team. Treatment along with dosage was clinically determined by the treatment team based on effectiveness of the drug and the patient’s tolerance. Healthy controls were administered the GHQ-12.
Patients repeated the YMRS at weeks 2 and 6. Blood samples were collected from patients in the morning after admission, as well as from the first-degree relatives and healthy controls. Follow-up blood samples of the same patients were collected after 2 weeks and 6 weeks of treatment. Blood (10 mL) was drawn under standardized conditions (sitting patient from cubital vein). After the blood was collected, it was allowed to clot in a serum separator tube at room temperature. It was centrifuged for approximately 5 minutes. The serum was aliquoted and stored at −80°C for later analysis. The blood samples were tested for serum levels of hs-CRP using immunoturbidimetric assay. The measured level was reported by a biochemist and recorded.
Data Analysis
Data were tabulated and analyzed using SPSS for Windows, version 25.0. Group differences of continuous variables were measured by independent Student t test. Group differences of categorical variables were computed by χ2 test. Analysis of variance (ANOVA) was applied for comparison of biochemical markers at baseline among patients, first-degree relatives, and healthy controls. Paired t test was applied for comparison of biochemical markers of patients at baseline and remission. Correlation between the clinical and biochemical variables was done with Pearson correlation test. A P value < .05 was considered significant.
RESULTS
Sociodemographic Variables of the 3 Groups
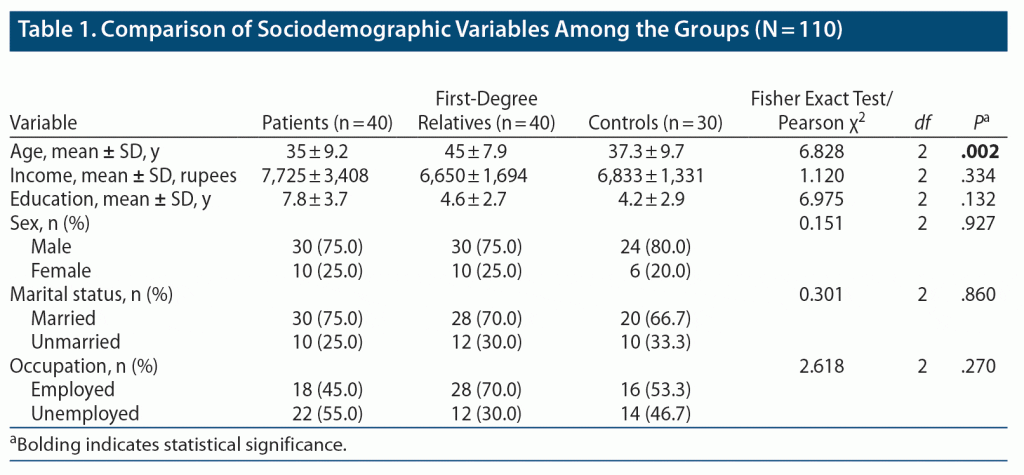
Table 1 compares the sociodemographic variables among the patients, first-degree relatives, and controls. All 3 groups were comparable in terms of sex, education, occupation, family income, and marital status. Most patients in this study were male, as were controls and first-degree relatives. All 3 groups had comparable monthly income, and there were no significant differences in education. Regarding age, first-degree relatives were older than the other 2 groups, and this difference was significant given that most first-degree relatives were parents or siblings who were older than the patients.
Comparison of Biochemical Parameter (hs-CRP Level) Among the 3 Groups at Baseline
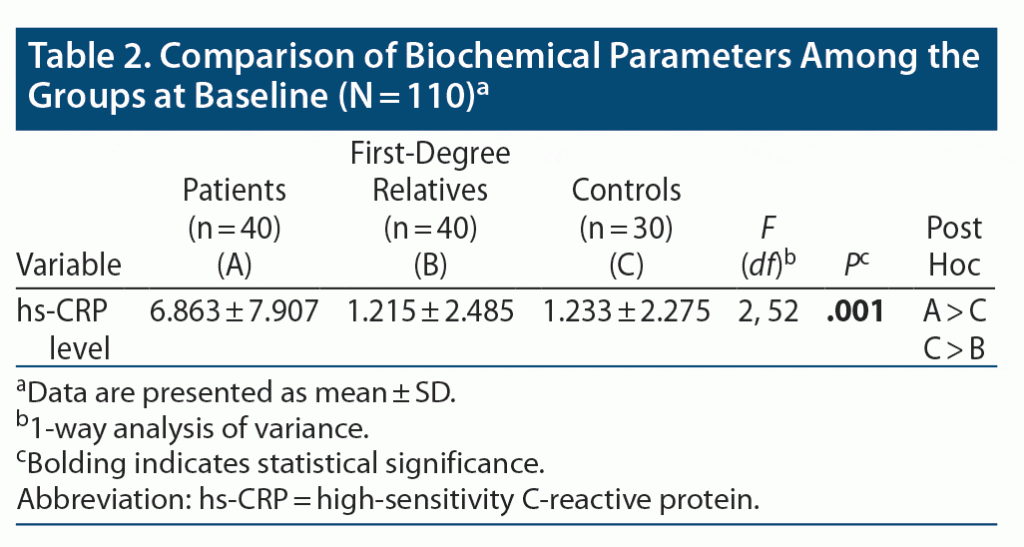
Table 2 shows a significant difference in the levels of hs-CRP between the patients and controls. The mean ± SD hs-CRP level was higher in patients (6.863 ± 7.907) than in controls (1.233 ± 2.275). There was a trend for hs-CRP levels to be higher in controls than in first-degree relatives. Measurement was conducted using 1-way ANOVA and post hoc Bonferroni correction.
Comparison of Changes in Biochemical and Clinical Parameters in Patients Over Time
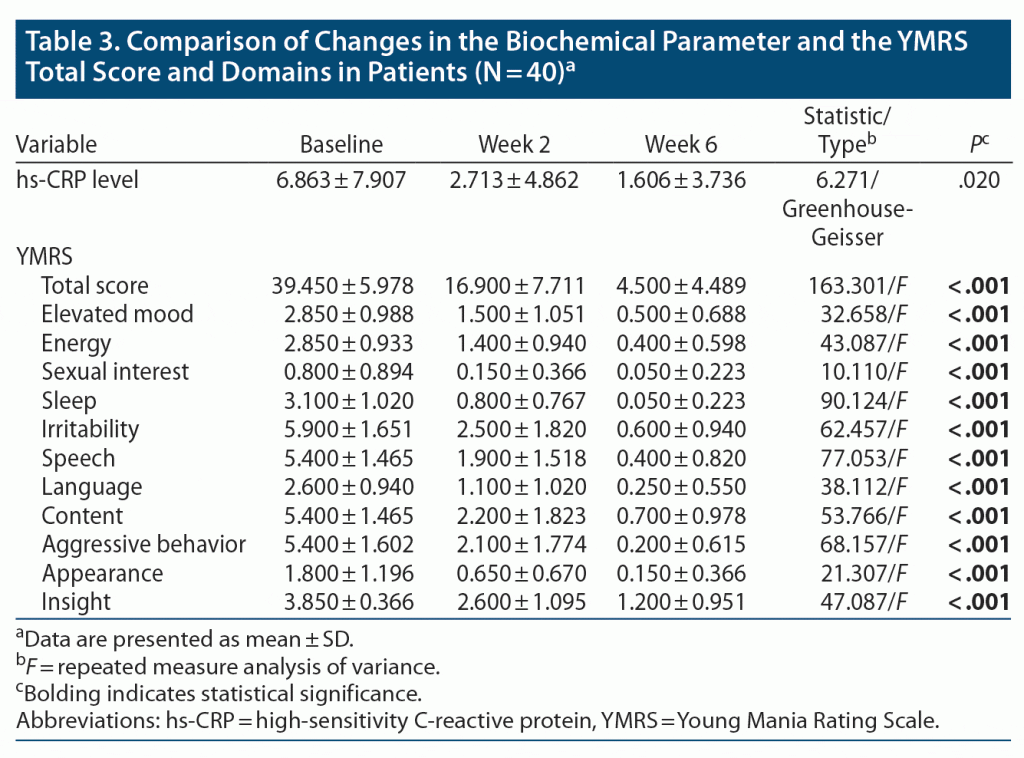
Table 3 compares the changes for hs-CRP in blood, as well as YMRS scores and its different domains over time in patients using repeated measures ANOVA. Significant changes with declining value were seen in 3 clinical observations with a P value of .020 and observed power of 0.637 in hs-CRP level. Regarding clinical parameters, there was a statistically significant decline in the YMRS total score and its domains over time from baseline to 6 weeks.
Correlation
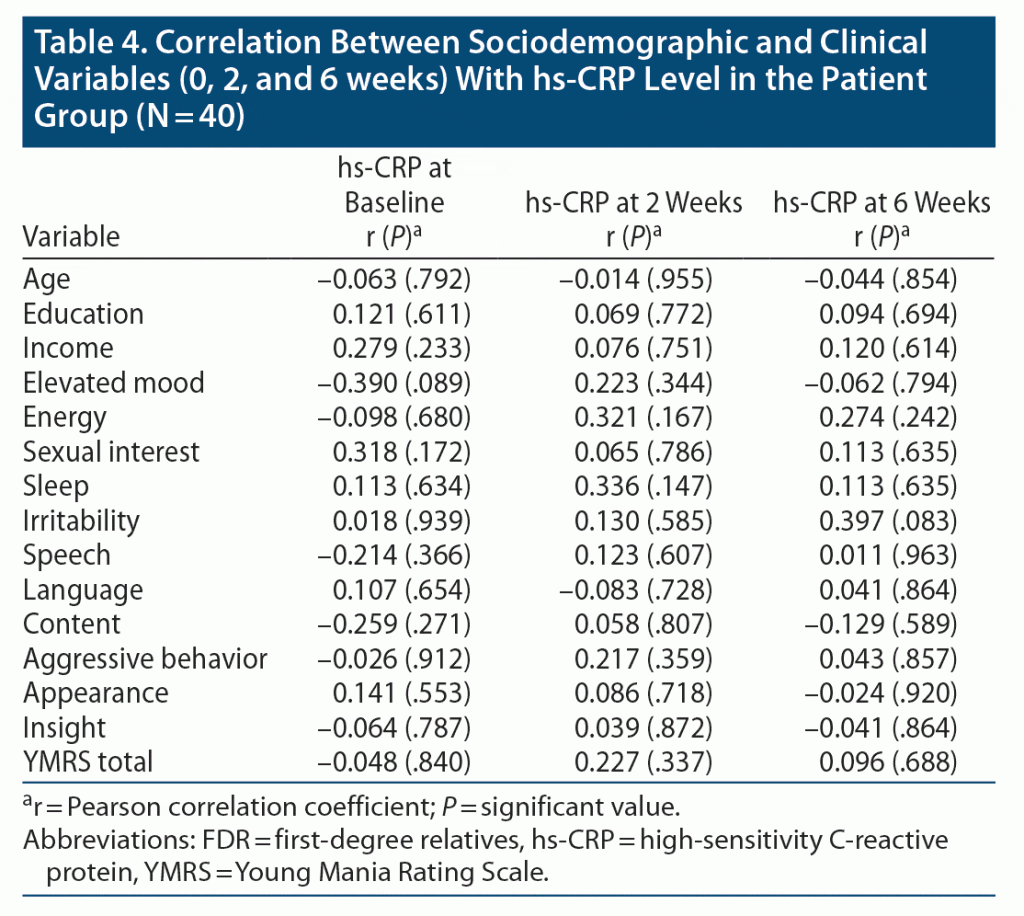
Table 4 shows the correlation between sociodemographic variables and hs-CRP levels taken at baseline, week 2, and week 6 in the patient group. No significant correlation was found between these 2 parameters. Regarding clinical parameters, the correlation was measured between YMRS total and domain scores at baseline, week 2, and week 6 with hs-CRP levels taken during the same period. No significant correlation was found between these parameters in the patient group.
DISCUSSION
Our study showed that hs-CRP levels were significantly higher in patients compared to controls and first-degree relatives. But levels were not higher in first-degree relatives compared to healthy controls. Although no significant correlation was found between sociodemographic variables, YMRS score, and hs-CRP levels in patients over time, the mean YMRS score and hs-CRP levels in patients were found to be significantly reduced at weeks 2 and 6 compared to baseline, indicating better treatment response over time. Thus, we found the hs-CRP level in blood to be a biomarker in mania, but whether it might be an early prodromal marker of developing disease or an early marker of disease response and an indicator for patient-specific treatment response was difficult to answer.
In our study, there was a significant difference in the levels of hs-CRP between the patients and controls. Thus, hs-CRP level was a marker of the disease state as compared to healthy controls. A previous study22 found that cytokines such as TNF-α, INF-γ, IL-6, and hs-CRP were significantly higher in patients with a manic episode of bipolar I disorder before treatment than in healthy controls. Another study23 found mean levels of CRP were higher in patients with mania then in healthy individuals, but no significant difference was found with other disease conditions like acute schizophrenia, unipolar depression, and bipolar depression.
In our study, there was a trend for the levels of hs-CRP to be higher in controls than in first-degree relatives. So, it was difficult to establish hs-CRP as an endophenotype according to our study findings. Although research on endophenotypes in bipolar disorder is growing and first-degree relatives have been recruited to investigate trait markers, little is understood about the possible interactions of biomarkers and their role as a trait marker of bipolar disorder, especially in the manic phase.24
Regarding the evaluation of changes in mean YMRS total score and domains and hs-CRP levels in the patient group, statistically significant changes were seen in 3 clinical observations in both parameters. These results indicate that there was a significant effect of treatment on the levels of hs-CRP and the manic symptoms. hs-CRP does appear to be a marker of disease state and response to therapy. This finding was in concordance with previous study findings in which hs-CRP was observed to be the only parameter correlated with clinical response.22
No significant correlation was found between hs-CRP levels and sociodemographic variables or YMRS total and domain scores over time from baseline to weeks 2 and 6 in the patient group. To our knowledge, no study has found any such correlation in mania patients, which requires further evaluation in future research.
Our study had a few limitations. The sample size was small, and the intervals between blood sample collections from patients were relatively short. The effect of drugs and their dosage on the biomarkers could be varied. So, a larger sample size with patients with mania from the community would have provided better generalization of the result. Using an objective measure for assessment of symptoms, uniform usage of medication and longer intervals between blood sample collections could have yielded a better result.
Submitted: November 20, 2021; accepted March 11, 2022.
Published online: December 6, 2022.
Relevant financial relationships: None.
Funding/support: None.
Clinical Points
- High-sensitivity C-reactive protein (hs-CRP) levels in the blood can be a useful biomarker in patients with mania.
- Treatment response in patients with mania can be assessed by measuring blood hs-CRP levels over time.
- The role of blood hs-CRP level as a trait marker in mania requires further research.
References (24)

- McNamara RK, Lotrich FE. Elevated immune-inflammatory signaling in mood disorders: a new therapeutic target? Expert Rev Neurother. 2012;12(9):1143–1161. PubMed CrossRef
- World Health Organization. Biomarkers in Risk Assessment: Validity and Validation. Environ Health Criteria; 222. WHO website. Accessed October 14, 2022. https://apps.who.int/iris/handle/10665/42363
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21(4):374–383. PubMed CrossRef
- Pasic J, Levy WC, Sullivan MD. Cytokines in depression and heart failure. Psychosom Med. 2003;65(2):181–193. PubMed CrossRef
- Goldstein BI, Kemp DE, Soczynska JK, et al. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009;70(8):1078–1090. PubMed CrossRef
- Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461(16):25–46. PubMed CrossRef
- Dickerson F, Stallings C, Origoni A, et al. Elevated serum levels of C-reactive protein are associated with mania symptoms in outpatients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(4):952–955. PubMed CrossRef
- Knight ML. The application of high-sensitivity c-reactive protein in clinical practice a 2015 update. US Pharm. 2015;40(2):50–53.
- Wadee AA, Kuschke RH, Wood LA, et al. Serological observations in patients suffering from acute manic episodes. Hum Psychopharmacol. 2002;17(4):175–179. PubMed CrossRef
- Huang TL, Lin FC. High-sensitivity C-reactive protein levels in patients with major depressive disorder and bipolar mania. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):370–372. CrossRef
- Cunha AB, Andreazza AC, Gomes FA, et al. Investigation of serum high-sensitive C-reactive protein levels across all mood states in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2008;258(5):300–304. PubMed CrossRef
- Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122(566):15–30. PubMed CrossRef
- Gottesman II, McGue M. Endophenotype. Encyclopedia Clin Psychol. 2014;12(3):1–8.
- De Berardis D, Conti CM, Campanella D, et al. Evaluation of C-reactive protein and total serum cholesterol in adult patients with bipolar disorder. Int J Immunopathol Pharmacol. 2008;21(2):319–324. PubMed CrossRef
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–435. PubMed CrossRef
- World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1992.
- Howes OD, Lim S, Theologos G, et al. A comprehensive review and model of putative prodromal features of bipolar affective disorder. Psychol Med. 2011;41(8):1567–1577. PubMed CrossRef
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. PubMed CrossRef
- Suarez EC, Lewis JG, Krishnan RR, et al. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29(9):1119–1128. PubMed CrossRef
- McDowell I. Measuring Health: A Guide to Rating Scales and Questionnaires. USA: Oxford University Press; 2006.
- Bali R, Sharma P, Ghanghas P, et al. To compare the efficacy of c-reactive protein and total leucocyte count as markers for monitoring the course of odontogenic space infections. J Maxillofac Oral Surg. 2017;16(3):322–327. PubMed CrossRef
- Uyanik V, Tuglu C, Gorgulu Y, et al. Assessment of cytokine levels and hs-CRP in bipolar I disorder before and after treatment. Psychiatry Res. 2015;228(3):386–392. PubMed CrossRef
- Wysokiński A, Margulska A, Strzelecki D, et al. Levels of C-reactive protein (CRP) in patients with schizophrenia, unipolar depression and bipolar disorder. Nord J Psychiatry. 2015;69(5):346–353. PubMed CrossRef
- Hope S, Hoseth E, Dieset I, et al. Inflammatory markers are associated with general cognitive abilities in schizophrenia and bipolar disorder patients and healthy controls. Schizophr Res. 2015;165(2–3):188–194. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top