ABSTRACT
Symptoms and disease pathophysiology of myasthenia gravis (MG) vary considerably with each patient, and their individual preferences and priorities add to the need for individualized treatment of this autoimmune disease. Research in MG has grown substantially in recent years. New treatments have the potential of being both effective and well tolerated, addressing the trade-off of choosing either efficacy or tolerability when selecting treatments. Promising investigational treatments that may become available in the future may allow more patients than ever before to achieve an asymptomatic state, with the ultimate goal being to turn off abnormal antibody production.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation.
This Academic Highlights section of The Primary Care Companion for CNS Disorders presents the highlights of the webinar “Disease Mechanisms and the Changing Treatment Landscape for Generalized Myasthenia Gravis,” which was held on January 22, 2022. This report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from Argenx.
The webinar was chaired by Vera Bril, MD, FRCPC, from the Division of Neurology, University of Toronto, and University Health Network. The faculty were Carolina Barnett-Tapia, MD, PhD, from the Division of Neurology, University of Toronto, and University Health Network; and Nicholas J. Silvestri, MD, FAAN, from the University at Buffalo Jacobs School of Medicine and Biomedical Sciences, New York.
CME Objectives
After studying this article, you should be able to:
- Consider mechanisms of disease and actions of medication in treatment decisions for patients with generalized myasthenia gravis (gMG) (including immunoglobulin G [IgG] and acetylcholine receptor [AChR] antibodies, fragment crystallizable neonatal receptor [FcRn] modulation)
- Counsel patients on the changing landscape of gMG treatment
- Incorporate evidence on efficacy and safety into the process of tailoring appropriate therapies for patients with gMG, both with current treatments and as novel ones become available
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Release, Expiration, and Review Dates
This educational activity was published in October 2022 and is eligible for AMA PRA Category 1 Credit through December 31, 2023. The latest review of this material was September 2022.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for AbbVie, Acadia, Allergan, Eisai, Merck, and Takeda; has been a stock shareholder of M-3 Information; and has received royalties from UpToDate and Oxford University Press. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears on the next page.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category Credit. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Nurses Credentialing Center (ANCC) and the American Academy of Physicians Assistants (AAPA) accept certificates of participation for educational activities certified for AMA PRA Category 1 Credit from organizations accredited by the ACCME.
Financial Disclosure
Dr Bril has received compensation for consulting from Grifols, CSL, UCB, Argenx, Takeda, Alnylam Octapharma, Pfizer, Powell Mansfield Inc, Akcea, Ionis Immunovant, Sanofi, Momenta (J&J), Roche, Janssen, AZ-Alexion, and NovoNordisk and received grant/research support from AZ-Alexion, Grifols, CSL, UCB, Argenx, Takeda, Octapharma, Akcea, Momenta (J&J), Immunovant, and Ionis. Dr Silvestri has received compensation for consulting from Argenx, Alexion, Immunovant, and UCB. Dr Barnett-Tapia has served as a consultant for Argenx and Alexion; received grant/research support from the US Department of Defense, MGNet, Muscular Dystrophy Canada, and Grifols; and served on the speaker/advisory board for Sanofi. No member of the CME Institute staff reported any relevant personal financial relationships.
Review Process
The faculty members agreed to provide a balanced and evidence-based presentation and discussed the topics and CME objectives during the planning sessions. The faculty’s submitted content was validated by CME Institute staff, and the activity was evaluated for accuracy, use of evidence, and fair balance by the Chair and a peer reviewer who is without conflict of interest.
The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or Argenx.
© 2022 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2022;00(0):AR21018AH3C
To cite: Bril V, Silvestri NJ, Barnett-Tapia C. Like a game of chess, every move matters: the role of antibodies in the myasthenia gravis treatment landscape. Prim Care Companion CNS Disord. 2022;00(0):AR21018AH3C.
To share: https://doi.org/10.4088/PCC.AR21018AH3C
© 2022 Physicians Postgraduate Press, Inc.
Treatment of myasthenia gravis (MG) is complicated and must be highly individualized based on patients’ varied symptoms and disease pathophysiology and their individual preferences and priorities. Until very recently, clinicians and patients have had to make difficult decisions whether to sacrifice either efficacy or tolerability when selecting treatments. New treatments have the potential of being both effective and well tolerated. The following Academic Highlights presents an overview of a recent webinar in which the faculty discussed the current treatment landscape for MG and promising investigational treatments that may become available in the future.
PRESENTATION, DIAGNOSIS, AND CURRENT TREATMENT
The incidence rate of MG is 5 to 30 cases per million,1 and the prevalence rate is 10 to 20 cases per 100,000 population.1 MG has a bimodal age distribution, with one peak in incidence occurring around age 30 (predominantly females) and a second around 50 or 60 years of age (slightly more predominant in males).1
Pathophysiology
Dr Silvestri identified MG as a prototypical autoimmune disease in which antibodies interfere with neuromuscular transmission, causing weakness.1 Three subtypes of MG have been identified, with a small percentage of patients who do not fall into one of these subtypes and are identified as seronegative.
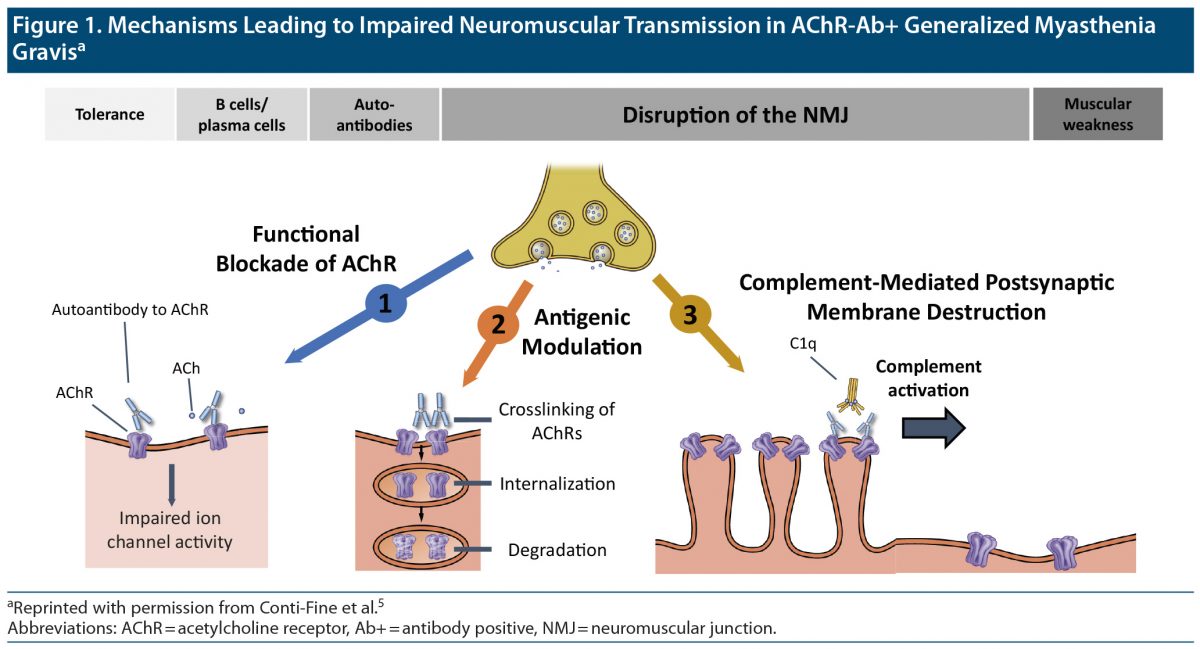
Acetylcholine receptor AChR) antibody positive (Ab+) MG. This is the most common subtype of MG.2 Patients with this subtype will test positive for autoantibodies against the AChR that belong to the immunoglobulin G (IgG) subtypes3 IgG1 and IgG3. These autoantibodies may originate in the thymus4 and interfere with nicotinic AChRs in the postsynaptic membrane of the neuromuscular junction through 3 main mechanisms.5 These are functional blockade,6 antigenic modulation,4 and complement activation (Figure 1).5
Muscle-specific tyrosine kinase (MuSK) Ab+ MG. approximately 7% of patients with MG7 are seropositive for MuSK autoantibodies, which are of the IgG4 subtype. MuSK autoantibodies do not cause complement activation, and patients with this subtype rarely exhibit abnormalities of the thymus.4
Low-density lipoprotein receptor-related protein 4 (LRP4) Ab+ MG. Autoantibodies against LRP4 are of the IgG1 subtype, which are capable of complement activation, thus leading to damage of the muscle end plate.8 It is also involved in activation of MuSK.
Clinical Presentation
MG leads to fatigable weakness,4 most commonly fatigability of the ocular muscles, leading to eyelid droop as well as double vision.4 Bulbar muscles are also affected, causing dysphagia, dysarthria, dysphonia, or chewing difficulty. Patients experience generalized weakness of the muscles of the neck and the proximal muscles of the limbs, though there are distal forms of MG.9 In serious cases, MG can lead to respiratory difficulties such as dyspnea, orthopnea, or respiratory failure, which is known as a myasthenic crisis.9
Diagnosis
A diagnosis of MG should be based on a thorough patient history that includes onset, course, and distribution of symptoms, as well as past medical and family history.10 Clinicians should test for fatigability and weakness through physical examination.10 Asking patients to stare at something to determine if diplopia or ptosis develop, applying an ice pack to test if ptosis improves,4 and pushing on hip and shoulder muscles until fatigue develops to assess proximal muscle fatigability can all indicate a diagnosis of MG. The Medical Research Council Scale for Muscle Strength can ensure uniformity of results and track changes over time.10
Assays are available that can detect antibodies of AChR, MuSK, and LRP4 receptors.11 In complicated cases or in seronegative cases, electrophysiologic tests such as repetitive nerve stimulation or single fiber electromyography can help with diagnosis.4 Edrophonium is a short-acting intravenous acetylcholinesterase inhibitor (AChEI) with a rapid onset of action that can be administered to test for improvement of weakness to confirm a diagnosis of MG.4
Patient Perspectives
A person describes being diagnosed with MG:
“When it comes to coping with a chronic illness, sometimes I think it’s almost easier after you’ve been diagnosed because the symptoms in advance of diagnosis can be very confusing. Once I formally had my senior diagnosis, I felt somewhat more confident in understanding how it would affect my life and what treatment options were available to me.”
Case Practice Question
Discussion of the best response can be found at the end of the activity.
Case 1. A 25-year-old woman presented with a 4-week history of intermittent diplopia, dysphagia, and dysarthria. Her examination is notable for fatigable ptosis in both eyelids and fatigable weakness proximally in her upper and lower extremities. Which of the following laboratory tests is most likely to lead to a diagnosis?
- Thyroid stimulating hormone
- Creatine kinase
- Antinuclear antibody
- Acetylcholine receptor antibody
Current Treatments
Available pharmacologic treatments include AChEIs, corticosteroids, oral immunosuppressants, intravenous immunoglobulin (IVIg), and plasmapheresis, and monoclonal antibodies have recently been developed to treat MG.
Thymectomy. Approximately 75%–85% of patients with MG12 have abnormalities of the thymus gland, and thymectomy is recommended for these individuals.9 Thymectomy has been associated with improved clinical outcomes and a reduced need for immunosuppressive therapies in patients with MG.13 Guidelines state that thymectomy should be offered to patients aged 18 to 50 years with AChR antibodies, but benefits and risks must be discussed.14
AChEIs. AChEIs are generally used as first-line treatment in patients who are AChR-Ab+, older than 50 years, or not good candidates for thymectomy.12 The most commonly used AChEI for MG is pyridostigmine.15 AChEIs will occasionally be adequate to control symptoms in mild cases of gMG, but for most patients adjunctive immunotherapy will be needed.2
Corticosteroids. Corticosteroids are first-line agents for immunosuppression and will likely be used at some point in most patients with MG.9,16 Most patients will achieve marked improvement or remission of symptoms within the first 2 to 4 weeks of corticosteroid treatment, with maximum benefit being achieved after 5 to 6 months of treatment.9 Because of the association with side effects, corticosteroids should be used in moderation and tapered carefully.17
Oral immunosuppressants. Oral immunosuppressants should be a first-line treatment for patients who are unable to take steroids, and they should be used as adjunctive treatment for patients who do not achieve adequate response to corticosteroid monotherapy.18 The most commonly used oral immunosuppressants are mycophenolate, azathioprine, tacrolimus, cyclosporine, and methotrexate.18
Second-line immunotherapy. IVIg and plasma exchange are both forms of immunotherapy generally used for short-term treatment, but these treatments can be used for extended periods in patients with refractory MG.18 These treatments have a rapid onset of effect and should be used in cases of myasthenic crisis.
Monoclonal antibodies (mAbs). Eculizumab is a mAb approved for the treatment of gMG.3 Eculizumab inhibits the complement pathway, preventing destruction of the postsynaptic membrane. Because MuSK antibodies do not activate the complement pathway, MuSK-Ab+ patients have not shown benefit with eculizumab treatment.19 The mAb rituximab has shown benefit in patients with both MuSK and AChR antibodies, although MuSK patients appear to experience greater benefit.3 Rituximab is thought to derive its therapeutic effect from its ability to deplete B cells. It is not approved for treatment of MG but is used off-label.17
CURRENT TREATMENT GAPS FOR MG
Intubation, ventilation, and thymectomy have improved outcomes dramatically in patients with MG, yielding a 10% mortality rate.20 However, according to Dr Barnett-Tapia, further progress is still needed.
Patient-Acceptable Symptom States
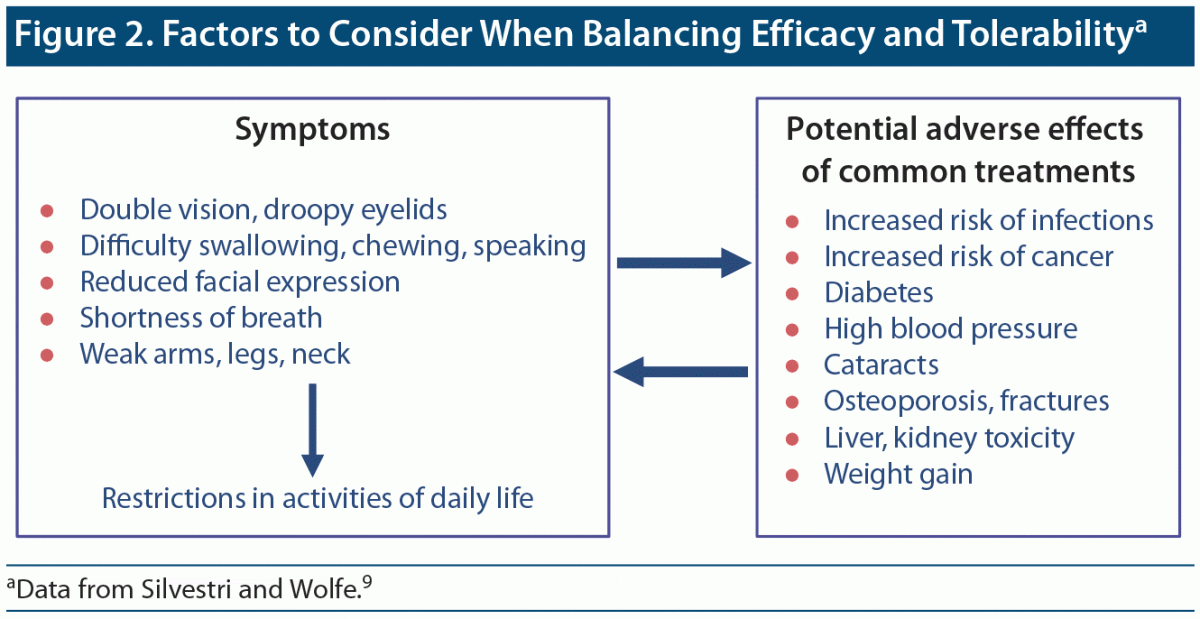
Most patients do get better with treatment,21–23 but the idea of “getting better” can be highly subjective. A more useful method of assessing improvement is to determine patient-acceptable symptom states. A large study conducted by Petersson and colleagues23 found that, despite receiving standard-of-care treatments, 47% of participants continued to experience an unacceptable burden of symptoms. Another survey found that approximately 30% of respondents reported unacceptable symptoms.22 According to Dr Barnett-Tapia, if symptoms improve but the patient remains unable to work, fulfill family responsibilities, and enjoy life, treatment has failed.
Treatment Trade-Offs
The balance between efficacy and tolerability must be evaluated for each patient because of individual disease course, patient lifestyles, demands, and comorbidities. Corticosteroids such as prednisone are effective in more than 80% of patients with MG, but two-thirds of patients9 experience weight gain, hypertension, diabetes, bone loss, and psychological disorders.9 Immunosuppressant therapies increase the risk of infections. A study24 compared infection rates over 5 years in 3,832 patients with MG and healthy comparators and found that MG was associated with a 39% greater risk of infection, including respiratory infections. Dr Barnett-Tapia pointed out that increased infection risk has become more troublesome during COVID-19.
Patient-Centered Treatment Goals
Qualitative studies exploring the experiences of individuals living with MG reveal that patients and providers often have different treatment priorities. For example, swallowing issues and respiratory issues can lead to hospital admissions, myasthenic crisis, and increased mortality, but patients may be more concerned with symptoms that impact their normal lives, such as double vision.
Symptom fluctuations. A recent study by Law and colleagues25 identified the unpredictable and fluctuating nature of symptoms as having a substantial negative impact on patients’ lives. Patients do not know if they will be able to complete their day of work or take their kids out to play. By understanding the individual’s burden due to symptom fluctuations, a clinician can prioritize a treatment that provides stability.
Patient Perspectives
Here, a person with MG describes her efforts to balance treatment efficacy and tolerability with her physician:
“As new treatments become available, we talk about them to get a sense of why there are medications that she is suggesting for patients or is not suggesting for patients and why they would be appropriate for me…. In particular with Dr Bril, I understand that her goal is not to overmedicate me. Her goal is to find the appropriate level of medication that lets me lead the best life that I can lead without overexposing me to the side effects of medication. And it’s a very delicate balance, and it’s something that I will probably continue balancing for the rest of my life.”
Treatment inertia. In the study by Laws et al,25 patients felt that their providers sometimes suffered from treatment inertia and were reluctant to try innovative or more aggressive treatments if the current treatment strategy had led to some improvement. Dr Barnett-Tapia suggested that treatment inertia may stem from worry of increasing side effects. In other instances, clinicians may want to delay making changes to give the current treatment sufficient time to take effect. Some treatments can take up to a year before any benefit is seen. However, patients feel as though they have wasted a year of their lives, especially if the treatment does not work. Ultimately, clinicians must remember that treatment decisions should be based on the patient’s goals and preferences.
Mental health. Approximately 41% of individuals with MG26 have comorbid mood disorders and other mental health problems, which often stem from the physical burden and unpredictable nature of MG symptoms. Unfortunately, mental health symptoms often remain untreated, which is an important treatment gap.26
Case Practice Question
Discussion of the best response can be found at the end of the activity.
Case 2. A 42-year-old woman with MG is currently treated with prednisone 10 mg per day, azathioprine 100 mg per day, and pyridostigmine 60 mg 3 times per day. While she has improved, she is still experiencing double vision that affects her ability to work, as well as severe fatigue. On examination, you see mild ptosis and an otherwise normal examination. She asks about counseling and treatment alternatives. Which alternatives are the most appropriate?
- You reassure her that her examination is essentially normal, and there is no need to change her treatment.
- You acknowledge that her disease is not well controlled and discuss the potential benefit and adverse events of increasing treatment doses.
- You explain that increasing the doses of her treatments conveys unacceptable risks for adverse events, and she should maintain treatment and adjust to her symptoms.
- You explain that azathioprine takes time to start working, and if there is no improvement in 6 months or she requires a higher dose of prednisone, there are other treatment options for her.
The ultimate goal of clinicians must be to find a balance between improving symptoms and helping patients achieve their best quality of life (Figure 2).
FC RECEPTOR INHIBITOR THERAPY
MG is an antibody-mediated disease in which the production of abnormal antibodies leads to a loss of tolerance of the AChR, and new Fc receptor (FcR) inhibitor therapies for MG target specific antibody mechanisms underlying MG.
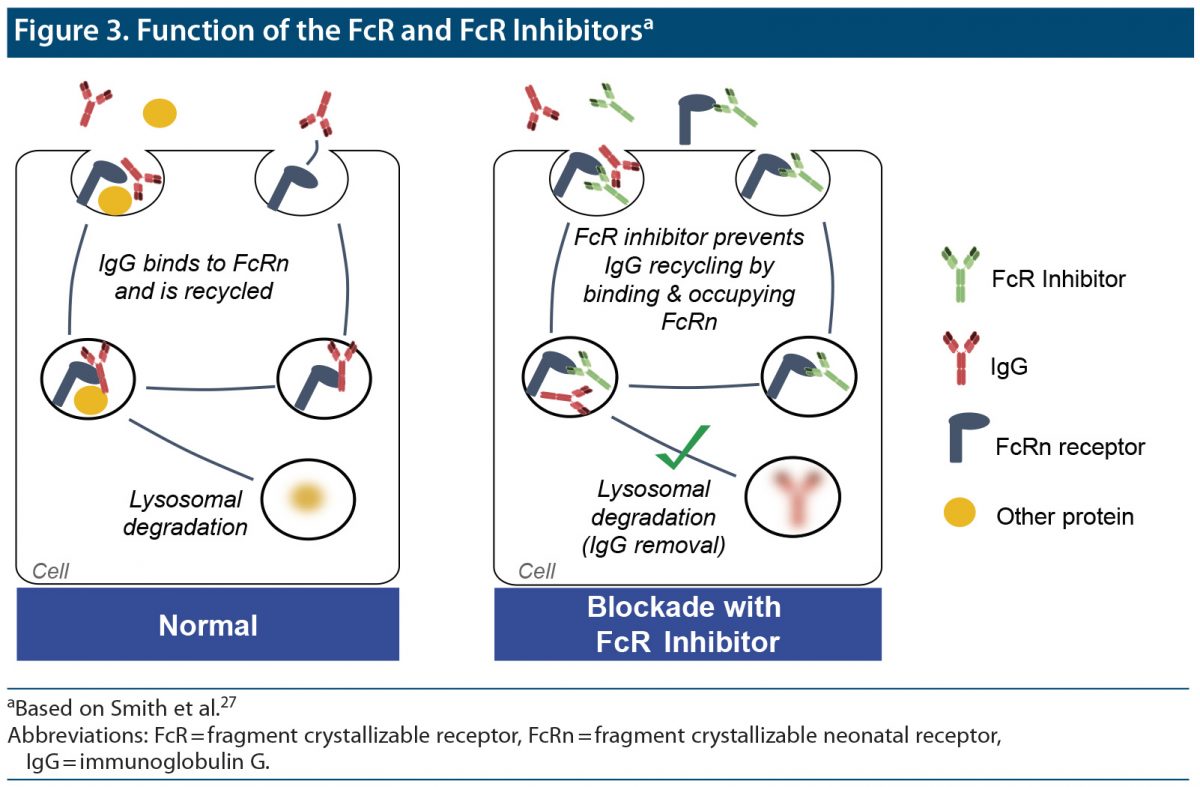
Fragment Crystallizable Neonatal Receptor (FcRn) Function and Inhibition
Normally, IgG binds to the FcRn and is then recirculated to the cell’s surface membrane. If the FcRn is inhibited, the IgG is delivered to lysosomes, where it is degraded rather than recycled. Thus, FcR inhibitors reduce IgG levels by blocking the recycling mechanism that maintains IgG levels in circulation, including the abnormal Ig activity at AChRs in MG.27 This type of therapy is effective in not only AChR-Ab+ cases, but also MuSK cases (Figure 3).27
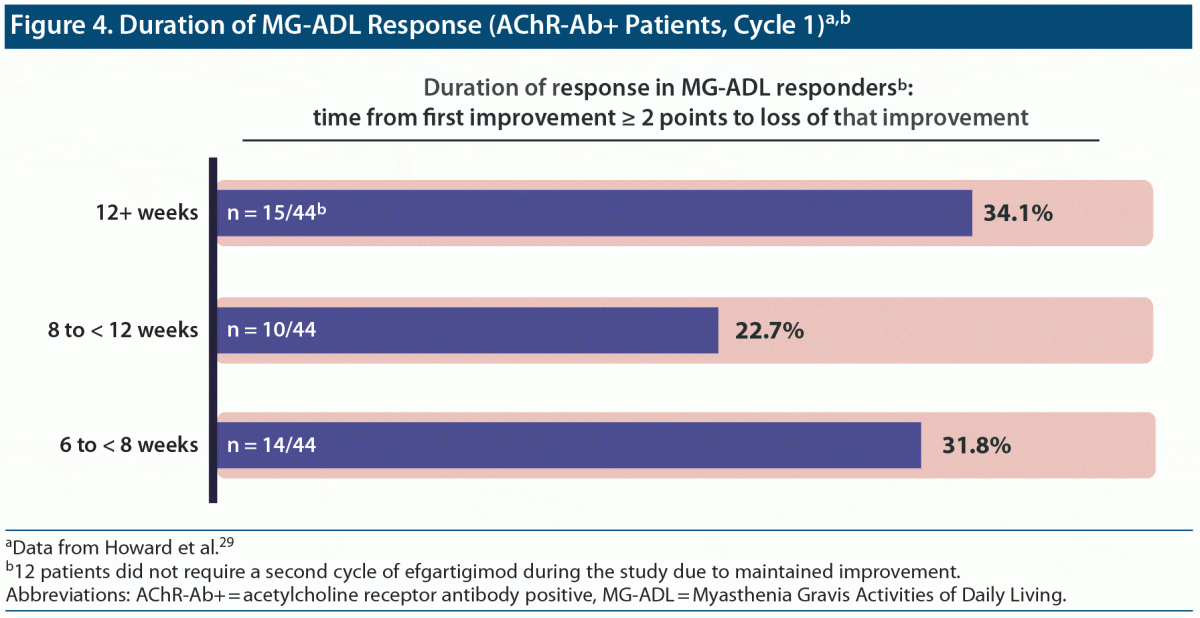
Efgartigimod. Efgartigimod is the only FcR inhibitor currently approved for MG.28 Approval was based on the results of the phase 3 ADAPT trial in which close to 68% of the efgartigimod group29 were MG-ADL responsive, and the second cycle showed that the response was repeatable.30 Minimal symptom expression (MG-specific Activities of Daily Living [ADL] scale ≥ 1) was achieved by 40% of patients.31 Treatment response endured for at least 6 weeks in more than 77% of patients, and the response lasted more than 12 weeks in more than one-third of patients.29 According to Dr Bril, the results of this study are promising, but the variability in duration of effect underscores the need to individualize treatment (Figure 4).
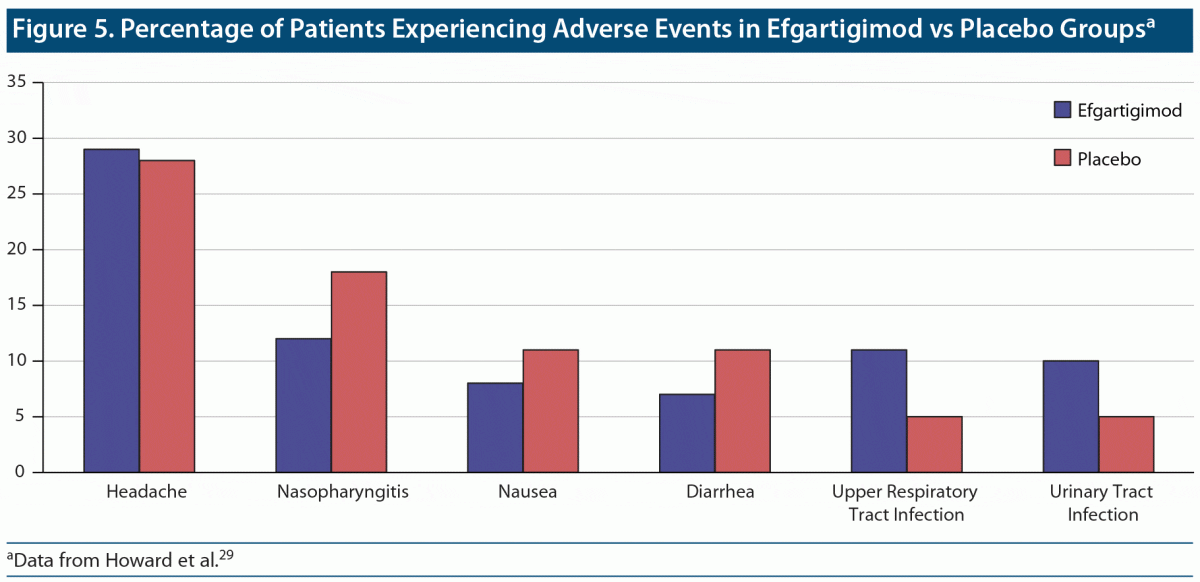
Overall, the ADAPT study found efgartigimod to be well tolerated, with adverse events being more common in the placebo group than in the efgartigimod group (84% vs 77%)29 (Figure 5).
Investigational FcR Inhibitors
A number of FcR inhibitors are currently in clinical trials for the treatment of MG.30 Nipocalimab32 and RVT-140133 have both completed phase 2 trials. Rozanolixizumab recently completed a phase 3 trial and announced positive results34 in December 2021. The phase 2 trial showed that compared with those receiving placebo, the patients receiving subcutaneous rozanolixizumab showed clinical benefit, but this positive change did not reach statistical significance.35 Rozanolixizumab was generally well tolerated.
Case Practice Question
Discussion of the best response can be found at the end of the activity.
Case 3. A 40-year-old woman was diagnosed with AChR-Ab+ MG 4 years ago. Despite therapy with thymectomy, pyridostigmine, corticosteroids, and mycophenolate mofetil 1.5 g twice daily, she has an inability to work, limited ability to care for her home and children, and difficulties with most ADL. She has been depressed over her continuing impairments and asks for better treatment.
Your next steps are to:
- Start a trial of IVIg followed by maintenance doses for 3 months and reassess. Continue with her other medications at present.
- Add FcR inhibitor efgartigimod to her treatment.
- Add eculizumab to her therapy.
- Any of the above.
CONCLUSION
Although many treatments are available for MG, patients continue to suffer as a result of inadequate symptom control or treatment side effects. Clinicians should be mindful of the trade-off that occurs between getting better and experiencing side effects. Ultimately, the cure will turn off abnormal antibody production. New treatments and those under investigation are promising and may allow more patients than ever before to achieve an asymptomatic state.
Clinical Points
- A diagnosis of myasthenia gravis should be based on evidence of fatigable weakness, patient history, and antibody testing.
- Treatments consist of thymectomy, acetylcholinesterase inhibitors, corticosteroids, immunosuppressants, and monoclonal antibodies.
- Treatment selection should be based on MG subtype and the patient’s needs and preferences.
- The efficacy of treatments must be weighed against the burden of potential side effects.
- FcR inhibitors are promising new treatments that appear to be highly effective and well tolerated.29
Discussion of Case Practice Questions
Case 1: Preferred response is d. Acetylcholine receptor antibody.
This case of fatigable ocular and bulbar-predominant weakness in a young woman is very suggestive of a diagnosis of MG. As 85% of patients with MG have AChR antibodies, this test is most likely to lead to a diagnosis in this case.
Case 2: Preferred response is b. You acknowledge that her disease is not well controlled and discuss the potential benefit and adverse events of increasing treatment doses and d. You explain that azathioprine takes time to start working, and if there is no improvement in 6 months or she requires a higher dose of prednisone, there are other treatment options for her.
Up to 40% of people with MG report having unacceptable symptom burden, despite treatment, and MG symptoms fluctuate. Treatment decisions should be individualized to assess specific risk/benefit profile of medications for each patient, including patients’ expectations, usual activities, and comorbidities. Many traditional immunosuppressants take approximately 12 months to act, so an appropriate time on treatment is needed before changing to other alternatives.
Case 3: Preferred response is d. Any of the above.
All of these therapies have shown a rapid onset of action and benefits to patients not doing well on other therapies. Eculizumab was tested specifically in refractory patients, so eculizumab is a good choice but very expensive. There is evidence that maintenance therapy with IVIg or subcutaneous infusion immunoglobulin can improve MG status and reduce doses of corticosteroids and immunosuppressants. The novel FcR inhibitor will act rapidly and improve this patient’s status.
Published online: Month 0, 2022.
Disclosure of off-label usage: The faculty of this educational activity may include discussions of products or devices that are not currently labeled for use by the FDA. Faculty members have been advised to disclose to the audience any reference to an unlabeled or investigational use. No endorsement of unapproved products or uses is made or implied by coverage of these products or uses. Please refer to the official prescribing information for each product for discussion of approved indicators, contraindications, and warnings.
REFERENCES
- 1. Hehir MK, Silvestri NJ. Generalized myasthenia gravis: classification, clinical presentation, natural history, and epidemiology. Neurol Clin. 2018;36(2):253–260. PubMed CrossRef
- 2. Angelini C. Diagnosis and management of autoimmune myasthenia gravis. Clin Drug Investig. 2011;31(1):1–14. PubMed CrossRef
- 3. Fichtner ML, Jiang R, Bourke A, et al. Autoimmune pathology in myasthenia gravis disease subtypes is governed by divergent mechanisms of immunopathology. Front Immunol. 2020;11:776. PubMed CrossRef
- 4. Li Y, Arora Y, Levin K. Myasthenia gravis: newer therapies offer sustained improvement. Cleve Clin J Med. 2013;80(11):711–721. PubMed CrossRef
- 5. Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116(11):2843–2854. PubMed CrossRef
- 6. Huijbers MG, Lipka AF, Plomp JJ, et al. Pathogenic immune mechanisms at the neuromuscular synapse: the role of specific antibody-binding epitopes in myasthenia gravis. J Intern Med. 2014;275(1):12–26. PubMed CrossRef
- 7. Rivner MH, Pasnoor M, Dimachkie M, et al. Muscle-specific tyrosine kinase and myasthenia gravis owing to other antibodies. Neurol Clin. 2018;36(2):293–310. PubMed CrossRef
- 8. Ha JC, Richman DP. Myasthenia gravis and related disorders: pathology and molecular pathogenesis. Biochim Biophys Acta. 2015;1852(4):651–657. PubMed CrossRef
- 9. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32(3):215–226. PubMed CrossRef
- 10. Amato AA. Approach to patients with neuromuscular disorders. Neuromuscular Disorders. 1–12.
- 11. Rousseff RT. Diagnosis of myasthenia gravis. J Clin Med. 2021;10(8):1736. PubMed CrossRef
- 12. Mantegazza R, Bonanno S, Camera G, et al. Current and emerging therapies for the treatment of myasthenia gravis. Neuropsychiatr Dis Treat. 2011;7:151–160. PubMed CrossRef
- 13. Wolfe GI, Kaminski HJ, Aban IB, et al; MGTX Study Group. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375(6):511–522. PubMed CrossRef
- 14. Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114–122. PubMed CrossRef
- 15. Sieb JP. Myasthenia gravis: an update for the clinician. Clin Exp Immunol. 2014;175(3):408–418. PubMed CrossRef
- 16. Menon D, Bril V. Pharmacotherapy of generalized myasthenia gravis with special emphasis on newer biologicals. Drugs. 2022;82(8):865–887. PubMed CrossRef
- 17. Menon D, Barnett C, Bril V. Novel treatments in myasthenia gravis. Front Neurol. 2020;11:538. https://www.frontiersin.org/articles/10.3389/fneur.2020.00538. Accessed August 16, 2022. PubMed CrossRef
- 18. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87(4):419–425. PubMed CrossRef
- 19. Schneider-Gold C, Gilhus NE. Advances and challenges in the treatment of myasthenia gravis. Ther Adv Neurol Disord. 2021;14:17562864211065406. PubMed CrossRef
- 20. Can you die from myasthenia gravis? Myasthenia-Gravis.com. https://myasthenia-gravis.com/living/complication-death. Updated August 2022. Accessed August 18, 2022.
- 21. Grob D, Brunner N, Namba T, et al. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141–149. PubMed CrossRef
- 22. Mendoza M, Tran C, Bril V, et al. Patient-acceptable symptom states in myasthenia gravis. Neurology. 2020;95(12):e1617–e1628. PubMed CrossRef
- 23. Petersson M, Feresiadou A, Jons D, et al. Patient-reported symptom severity in a nationwide myasthenia gravis cohort: cross-sectional analysis of the Swedish GEMG Study. Neurology. 2021;97(14):e1382–e1391. PubMed CrossRef
- 24. Kassardjian CD, Widdifield J, Paterson JM, et al. Serious infections in patients with myasthenia gravis: population-based cohort study. Eur J Neurol. 2020;27(4):702–708. PubMed CrossRef
- 25. Law N, Davio K, Blunck M, et al. The lived experience of myasthenia gravis: a patient-led analysis. Neurol Ther. 2021;10(2):1103–1125. PubMed CrossRef
- 26. Law C, Flaherty CV, Bandyopadhyay S. A review of psychiatric comorbidity in myasthenia gravis. Cureus. 2020;12(7):e9184. PubMed CrossRef
- 27. Smith B, Kiessling A, Lledo-Garcia R, et al. Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration. MAbs. 2018;10(7):1111–1130. PubMed CrossRef
- 28. FDA Approves New Treatment for Myasthenia Gravis. US Food and Drug Administration. cements/fda-approves-new-treatment-myasthenia-gravis. Published December 17, 2021. Accessed May 31, 2022.
- 29. Howard JF Jr, Bril V, Vu T, et al; ADAPT Investigator Study Group. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20(7):526–536. PubMed CrossRef
- 30. Dalakas MC, Spaeth PJ. The importance of FcRn in neuro-immunotherapies: From IgG catabolism, FCGRT gene polymorphisms, IVIg dosing and efficiency to specific FcRn inhibitors. Ther Adv Neurol Disord.
- 31. Argenx Reports Positive Data of Efgartigimod in Myasthenia Gravis Trial. Clinical Trials Arena. https://www.clinicaltrialsarena.com/news/argenx-efgartigimod-phaseiii-data/. Published June 17, 2021. Accessed August 22, 2022. 2021;14:1756286421997381. PubMed CrossRef
- 32. Momenta Pharmaceuticals, Inc. A Phase 2, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Tolerability, Efficacy, Pharmacokinetics and Pharmacodynamics of M281 Administered to Adults With Generalized Myasthenia Gravis. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03772587. 2021. Accessed May 30, 2022.
- 33. Immunovant Sciences GmbH. A Phase 2a, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study With an Open-Label Extension of RVT-1401 in Myasthenia Gravis Patients. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03863080. 2021. Accessed May 30, 2022.
- 34. UCB Biopharma SRL. An Open-Label Extension Study to Evaluate Rozanolixizumab in Study Participants With Generalized Myasthenia Gravis. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04650854. 2022. Accessed May 30, 2022.
- 35. Bril V, Benatar M, Andersen H, et al; MG0002 Investigators. Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis: a phase 2 randomized control trial. Neurology. 2021;96(6):e853–e865. PubMed CrossRef
References (35)

- Hehir MK, Silvestri NJ. Generalized myasthenia gravis: classification, clinical presentation, natural history, and epidemiology. Neurol Clin. 2018;36(2):253–260. PubMed CrossRef
- Angelini C. Diagnosis and management of autoimmune myasthenia gravis. Clin Drug Investig. 2011;31(1):1–14. PubMed CrossRef
- Fichtner ML, Jiang R, Bourke A, et al. Autoimmune pathology in myasthenia gravis disease subtypes is governed by divergent mechanisms of immunopathology. Front Immunol. 2020;11:776. PubMed CrossRef
- Li Y, Arora Y, Levin K. Myasthenia gravis: newer therapies offer sustained improvement. Cleve Clin J Med. 2013;80(11):711–721. PubMed CrossRef
- Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116(11):2843–2854. PubMed CrossRef
- Huijbers MG, Lipka AF, Plomp JJ, et al. Pathogenic immune mechanisms at the neuromuscular synapse: the role of specific antibody-binding epitopes in myasthenia gravis. J Intern Med. 2014;275(1):12–26. PubMed CrossRef
- Rivner MH, Pasnoor M, Dimachkie M, et al. Muscle-specific tyrosine kinase and myasthenia gravis owing to other antibodies. Neurol Clin. 2018;36(2):293–310. PubMed CrossRef
- Ha JC, Richman DP. Myasthenia gravis and related disorders: pathology and molecular pathogenesis. Biochim Biophys Acta. 2015;1852(4):651–657. PubMed CrossRef
- Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32(3):215–226. PubMed CrossRef
- Amato AA. Approach to patients with neuromuscular disorders. Neuromuscular Disorders. 1–12.
- Rousseff RT. Diagnosis of myasthenia gravis. J Clin Med. 2021;10(8):1736. PubMed CrossRef
- Mantegazza R, Bonanno S, Camera G, et al. Current and emerging therapies for the treatment of myasthenia gravis. Neuropsychiatr Dis Treat. 2011;7:151–160. PubMed CrossRef
- Wolfe GI, Kaminski HJ, Aban IB, et al; MGTX Study Group. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375(6):511–522. PubMed CrossRef
- Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114–122. PubMed CrossRef
- Sieb JP. Myasthenia gravis: an update for the clinician. Clin Exp Immunol. 2014;175(3):408–418. PubMed CrossRef
- Menon D, Bril V. Pharmacotherapy of generalized myasthenia gravis with special emphasis on newer biologicals. Drugs. 2022;82(8):865–887. PubMed CrossRef
- Menon D, Barnett C, Bril V. Novel treatments in myasthenia gravis. Front Neurol. 2020;11:538. https://www.frontiersin.org/articles/10.3389/fneur.2020.00538. Accessed August 16, 2022. PubMed CrossRef
- Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87(4):419–425. PubMed CrossRef
- Schneider-Gold C, Gilhus NE. Advances and challenges in the treatment of myasthenia gravis. Ther Adv Neurol Disord. 2021;14:17562864211065406. PubMed CrossRef
- Can you die from myasthenia gravis? Myasthenia-Gravis.com. https://myasthenia-gravis.com/living/complication-death. Updated August 2022. Accessed August 18, 2022.
- Grob D, Brunner N, Namba T, et al. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141–149. PubMed CrossRef
- Mendoza M, Tran C, Bril V, et al. Patient-acceptable symptom states in myasthenia gravis. Neurology. 2020;95(12):e1617–e1628. PubMed CrossRef
- Petersson M, Feresiadou A, Jons D, et al. Patient-reported symptom severity in a nationwide myasthenia gravis cohort: cross-sectional analysis of the Swedish GEMG Study. Neurology. 2021;97(14):e1382–e1391. PubMed CrossRef
- Kassardjian CD, Widdifield J, Paterson JM, et al. Serious infections in patients with myasthenia gravis: population-based cohort study. Eur J Neurol. 2020;27(4):702–708. PubMed CrossRef
- Law N, Davio K, Blunck M, et al. The lived experience of myasthenia gravis: a patient-led analysis. Neurol Ther. 2021;10(2):1103–1125. PubMed CrossRef
- Law C, Flaherty CV, Bandyopadhyay S. A review of psychiatric comorbidity in myasthenia gravis. Cureus. 2020;12(7):e9184. PubMed CrossRef
- Smith B, Kiessling A, Lledo-Garcia R, et al. Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration. MAbs. 2018;10(7):1111–1130. PubMed CrossRef
- FDA Approves New Treatment for Myasthenia Gravis. US Food and Drug Administration. cements/fda-approves-new-treatment-myasthenia-gravis. Published December 17, 2021. Accessed May 31, 2022.
- Howard JF Jr, Bril V, Vu T, et al; ADAPT Investigator Study Group. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20(7):526–536. PubMed CrossRef
- Dalakas MC, Spaeth PJ. The importance of FcRn in neuro-immunotherapies: From IgG catabolism, FCGRT gene polymorphisms, IVIg dosing and efficiency to specific FcRn inhibitors. Ther Adv Neurol Disord.
- Argenx Reports Positive Data of Efgartigimod in Myasthenia Gravis Trial. Clinical Trials Arena. https://www.clinicaltrialsarena.com/news/argenx-efgartigimod-phaseiii-data/. Published June 17, 2021. Accessed August 22, 2022. 2021;14:1756286421997381. PubMed CrossRef
- Momenta Pharmaceuticals, Inc. A Phase 2, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Tolerability, Efficacy, Pharmacokinetics and Pharmacodynamics of M281 Administered to Adults With Generalized Myasthenia Gravis. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03772587. 2021. Accessed May 30, 2022.
- Immunovant Sciences GmbH. A Phase 2a, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study With an Open-Label Extension of RVT-1401 in Myasthenia Gravis Patients. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03863080. 2021. Accessed May 30, 2022.
- UCB Biopharma SRL. An Open-Label Extension Study to Evaluate Rozanolixizumab in Study Participants With Generalized Myasthenia Gravis. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04650854. 2022. Accessed May 30, 2022.
- Bril V, Benatar M, Andersen H, et al; MG0002 Investigators. Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis: a phase 2 randomized control trial. Neurology. 2021;96(6):e853–e865. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top