Objective: To assess the effects of the combination of SAMe (S-adenosylmethionine) 200 mg and Lactobacillus plantarum (L. plantarum) HEAL9 1 ×— 109 CFU for the overall symptomatology of mild-to-moderate depression.
Methods: This 6-week randomized, double-blind, placebo-controlled study included subjects aged 18-60 years with mild-to-moderate depression (according to ICD-10 diagnostic criteria) recruited from September 17, 2018, to October 5, 2018. Difference between groups in change from baseline to treatment week 6 on the Zung Self-Rating Depression Scale (Z-SDS) was the primary outcome. Comparisons between groups in change from baseline to treatment week 2 of the Z-SDS and from baseline to treatment weeks 2 and 6 of other scales (related to insomnia, anxiety, irritable bowel syndrome, and health status) were also analyzed.
Results: Ninety patients were randomized to SAMe plus L. plantarum HEAL9 (n = 46) or placebo (n = 44) groups. A greater reduction for the new combination compared to placebo was seen at treatment week 6 in the Z-SDS total score (P = .0165) and the core depression subdomain (P = .0247). A significant reduction in favor of the combination was shown at treatment week 2 for the Z-SDS total score (P = .0330), the cognitive and anxiety subdomains (P = .0133 and P = .0459, respectively), and the anxiety questionnaire (P = .0345). No treatment-related adverse events occurred.
Conclusions: Supplementation of SAMe and L. plantarum HEAL9 in adults with subthreshold or mild-to-moderate symptoms of depression resulted in fast and clinically relevant effects after 2 weeks. The combination was safe and significantly improved symptoms of depression, anxiety, and cognitive and somatic components. The effect of this novel product is independent from the severity of the symptoms unlike traditional antidepressants available on the market that have minimal benefits for subthreshold or mild-to-moderate symptoms.
Trial Registration: ClinicalTrials.gov identifier: NCT03932474
aheadofprint=’article’;
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Oral Administration of S-Adenosylmethionine (SAMe) and Lactobacillus Plantarum HEAL9 Improves the Mild-To-Moderate Symptoms of Depression:
A Randomized, Double-Blind, Placebo-Controlled Study
ABSTRACT
Objective: To assess the effects of the combination of SAMe (S-adenosylmethionine) 200 mg and Lactobacillus plantarum (L. plantarum) HEAL9 1 ×— 109 CFU for the overall symptomatology of mild-to-moderate depression.
Methods: This 6-week randomized, double-blind, placebo-controlled study included subjects aged 18-60 years with mild-to-moderate depression (according to ICD-10 diagnostic criteria) recruited from September 17, 2018, to October 5, 2018. Difference between groups in change from baseline to treatment week 6 on the Zung Self-Rating Depression Scale (Z-SDS) was the primary outcome. Comparisons between groups in change from baseline to treatment week 2 of the Z-SDS and from baseline to treatment weeks 2 and 6 of other scales (related to insomnia, anxiety, irritable bowel syndrome, and health status) were also analyzed.
Results: Ninety patients were randomized to SAMe plus L. plantarum HEAL9 (n = 46) or placebo (n = 44) groups. A greater reduction for the new combination compared to placebo was seen at treatment week 6 in the Z-SDS total score (P = .0165) and the core depression subdomain (P = .0247). A significant reduction in favor of the combination was shown at treatment week 2 for the Z-SDS total score (P = .0330), the cognitive and anxiety subdomains (P = .0133 and P = .0459, respectively), and the anxiety questionnaire (P = .0345). No treatment-related adverse events occurred.
Conclusions: Supplementation of SAMe and L. plantarum HEAL9 in adults with subthreshold or mild-to-moderate symptoms of depression resulted in fast and clinically relevant effects after 2 weeks. The combination was safe and significantly improved symptoms of depression, anxiety, and cognitive and somatic components. The effect of this novel product is independent from the severity of the symptoms unlike traditional antidepressants available on the market that have minimal benefits for subthreshold or mild-to-moderate symptoms.
Trial Registration: ClinicalTrials.gov identifier: NCT03932474
Prim Care Companion CNS Disord 2020;22(4):19m02578
To cite: Saccarello A, Montarsolo P, Massardo I, et al. Oral administration of S-adenosylmethionine (SAMe) and Lactobacillus plantarum HEAL9 improves the mild-to-moderate symptoms of depression: a randomized, double-blind, placebo-controlled study. Prim Care Companion CNS Disord. 2020;22(4):19m02578.
To share: https://doi.org/10.4088/PCC.19m02578
© Copyright 2020 Physicians Postgraduate Press, Inc.
aPrivate practice, Genova, Italy
bHippocrates Research SRL, Genova, Italy
cNutrilinea SRL, Varese, Italy
*Corresponding author: Irene Schiavetti, PhD, Hippocrates Research SRL, Via XX Settembre, 30/12, 16121 Genova, Italy ([email protected]).
Major depressive disorder substantially contributes to global disease burden given that it is estimated to affect 4.4% of the world population.1 The moderate-severe levels of depression, which are frequent in primary care, account for two-thirds of the total number (322 million) of cases.1 The diagnosis of major depressive disorder is based on objective criteria. For example, the International Classification of Diseases, Tenth Revision (ICD-10)2,3 categorizes depressive episodes into different groups according to the number of symptoms present, distinguishing between mild, moderate, and severe depression. A similar approach, with some differences, is followed by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).4
However, often, patients with depressive symptoms observed in clinical practice do not reach the minimum diagnostic criteria for major depressive disorder and therefore are described as having minor, subthreshold, or subclinical depression. Although this condition can significantly interfere with daily life, the therapeutic strategies to manage such groups of patients remain mostly undefined.5,6
Although antidepressant drugs such as tricyclic antidepressants (TCAs) or selective serotonin reuptake inhibitors (SSRIs) are considered to be standard of care for depression treatment and superior to placebo, their use in subthreshold depression is controversial.7 Moreover, the effect size of antidepressant drugs is strictly associated with the baseline severity of symptoms, with significant improvements in patients with severe depression and minimal changes in those with subthreshold mild-to-moderate levels.8
Food supplements or probiotics have been studied as adjunctive therapy to antidepressant drugs and psychotherapy for mild-to-moderate forms of depression.9 Among the studied supplements, the effect of the putative antidepressant S-adenosylmethionine (SAMe) may be due to its capability to attenuate oxidative and nitrosative stress that is partially similar to that of SSRIs.10,11
The modulation of the affective and cognitive function of the central nervous system has gained interest in recent years due to extensive studies of microbiota even in patients with major depressive disorder.12,13 In previous randomized, double-blind, placebo-controlled studies,14,15 the probiotic bacteria Lactobacillus plantarum (L. plantarum) 299v displayed a significant effect compared with placebo on stress markers such as cortisol levels. L. plantarum 299v has also been shown to significantly decrease symptoms of irritable bowel syndrome.16-18
This 6-week randomized, double-blind, placebo-controlled study aimed to investigate the effects of the oral combination of SAMe and L. plantarum HEAL9, a probiotic strain similar to L. plantarum 299v, on patients with subthreshold or mild-to-moderate depressive symptoms.
The primary objective of this study was to assess the efficacy of SAMe plus L. plantarum HEAL9 versus placebo in improving the overall symptomatology of depression after 6 weeks of treatment in subjects with mild-to-moderate depressive symptoms. This objective was evaluated by comparing the absolute change in the total Zung Self-Rating Depression Scale (Z-SDS)19 score from baseline to the end of treatment between the 2 groups. In the subgroup treated with the new combination, an explorative analysis was conducted to assess if the level of improvement in patients’ symptoms at the end of treatment was dependent on the initial severity of the disease.
The secondary objectives of the study were to evaluate and compare between groups the effects of treatment on symptoms associated with depression, including anxiety, sleep disturbance, and abdominal discomfort. Furthermore, the overall health status and the assessment of tolerability and safety were also investigated.
METHODS
Study subjects were recruited from 10 Italian general practitioners (GPs). The study protocol, the informed consent document, and all types of subject recruitment information were reviewed and approved by the Independent Ethics Committee for Nonpharmacologic Clinical Investigations in Genova, Italy (approval number 2018/08).
The investigators obtained written consent from each subject before performing any protocol-related activities. The study was registered at ClinicalTrials.gov (identifier: NCT03932474). Patient recruitment took place from September 17, 2018, to October 5, 2018.
Study Design and Patient Selection
Subjects who met the following criteria were enrolled in the study: men and women aged 18-60 years with signed and dated written informed consent; diagnosis of recurrent mild-to-moderate depressive disorders according to ICD-10/F33 criteria; Z-SDS raw score between 41 and 55; and ability to comply with the requirements of the entire study.
Subjects were excluded if they met any of the following criteria: pregnant or breast-feeding women, presence of ≥ 1 psychiatric disturbances (alcoholism, substance abuse, or dependency disorder; bipolar disorder; schizophrenia; or other personality disorder), treatment with psychotropic drugs (antipsychotics, anxiolytics, hypnotics, or sedatives), or oral consumption of food supplements (only multivitamins, salts, and trace elements were accepted).
Study Assessments
At baseline, patients with symptoms of mild-to-moderate depression according to ICD-10 diagnostic criteria were asked to complete the Z-SDS questionnaire and were selected for the study if their total score was between 41 and 55. Eligible subjects were then randomized (ratio 1:1) to receive either SAMe plus L. plantarum HEAL9 (1 ×— 109 CFU/dose) or placebo over a period of 6 weeks (42 consecutive days) following a computerized randomization list previously prepared for each GP. A block randomization (block size = 4) ensured a balance in treatment assignment over time. The GPs allocated a treatment number according to the chronological order of arrival of each subject at baseline. Both the patient and the GP were blinded to the assigned treatment. During the study, the GP had a set of individual sealed decoding cards, each corresponding to a treatment number in case unblinding would be needed due to a serious, product-related adverse event.
Demographic data and details regarding medical history, physical examination, and any intake of previous or concomitant medication were collected. Subjects also completed a booklet of the following questionnaires: Zung Self-Rating Anxiety Scale (Z-SAS),19 Insomnia Severity Index (ISI),20 Birmingham IBS symptom questionnaire (B-IBS),21 and EQ-5D-3L.22,23 All questionnaires were administered at the intermediate visit (day 14 ± 2) and at the end of the study (day 42 ± 2). During the last visit, subjects had to return unused products.
Zung Self-Rating Depression Scale
The Z-SDS is the questionnaire adopted for the evaluation of the primary endpoint.24 It is a norm-referenced self-administered scale widely used for measuring depression levels. The Z-SDS consists of 20 items that explore psychological and physiologic symptoms rated by the subjects according to how each applied to them within the past week by a 4-point Likert scale ranging from 1 (none or a little of the time) to 4 (most or all of the time). Items have been selected on the basis of previous factor analytic studies of depression symptoms. The Z-SDS has been implemented in clinical research related to behaviors and mental disorders for the evaluation of depression levels over time.25
Interventions
Subjects received an oral tablet formulation containing SAMe 200 mg and L. plantarum HEAL9 1 ×— 109 CFU or an inert oral tablet formulation (placebo). The 2 tablets were indistinguishable (ie, identical in shape, size, color, and taste). At baseline, each subject received 1 kit containing 45 tablets and directly ingested the first tablet. Subjects took 1 tablet daily for 42 consecutive days with a small amount of water, preferably without food.
Statistical Analysis
A sample size of 88 subjects (44 per group) was calculated to achieve 80% power to detect a 6-point difference in the absolute change from baseline to week 6 between groups with a significance level (α) of 0.05 using a 2-sample t-test. The size effect was established considering the difference in mean ± SD Z-SDS score between “nondepressed subjects” according to DSM criteria (46.8 ± 8.8) and “depressed subjects” (53.7 ± 8.2).26 Three different analysis sets were planned:
- Intention-to-treat (ITT): all randomized patients to whom the study treatment is dispensed and with at least 1 nonmissing postbaseline assessment in the primary variable;
- Per protocol (PP): all patients in the ITT population who completed the study fully compliant with the protocol and with no major violation/deviation;
- Safety population: all randomized patients with at least 1 documented intake of any study treatment.
Data were summarized as mean with SD and median with range (minimum and maximum) for continuous variables and absolute and relative frequency for categorical variables. The analysis of covariance (ANCOVA) was used for primary and secondary efficacy analysis, with treatment and center (GPs) as an independent factor, baseline measure as a covariate, and change from baseline as a dependent variable. Results were reported as least-square means for treatment differences with associated 2-tailed 95% confidence intervals and corresponding 2-sided P values.
Missing data on the primary efficacy endpoint were assigned using multiple imputations as the primary imputation method and with last observation carried forward (LOCF) as a sensitivity analysis. Missing data on all the secondary efficacy endpoints were imputed only using the LOCF approach.
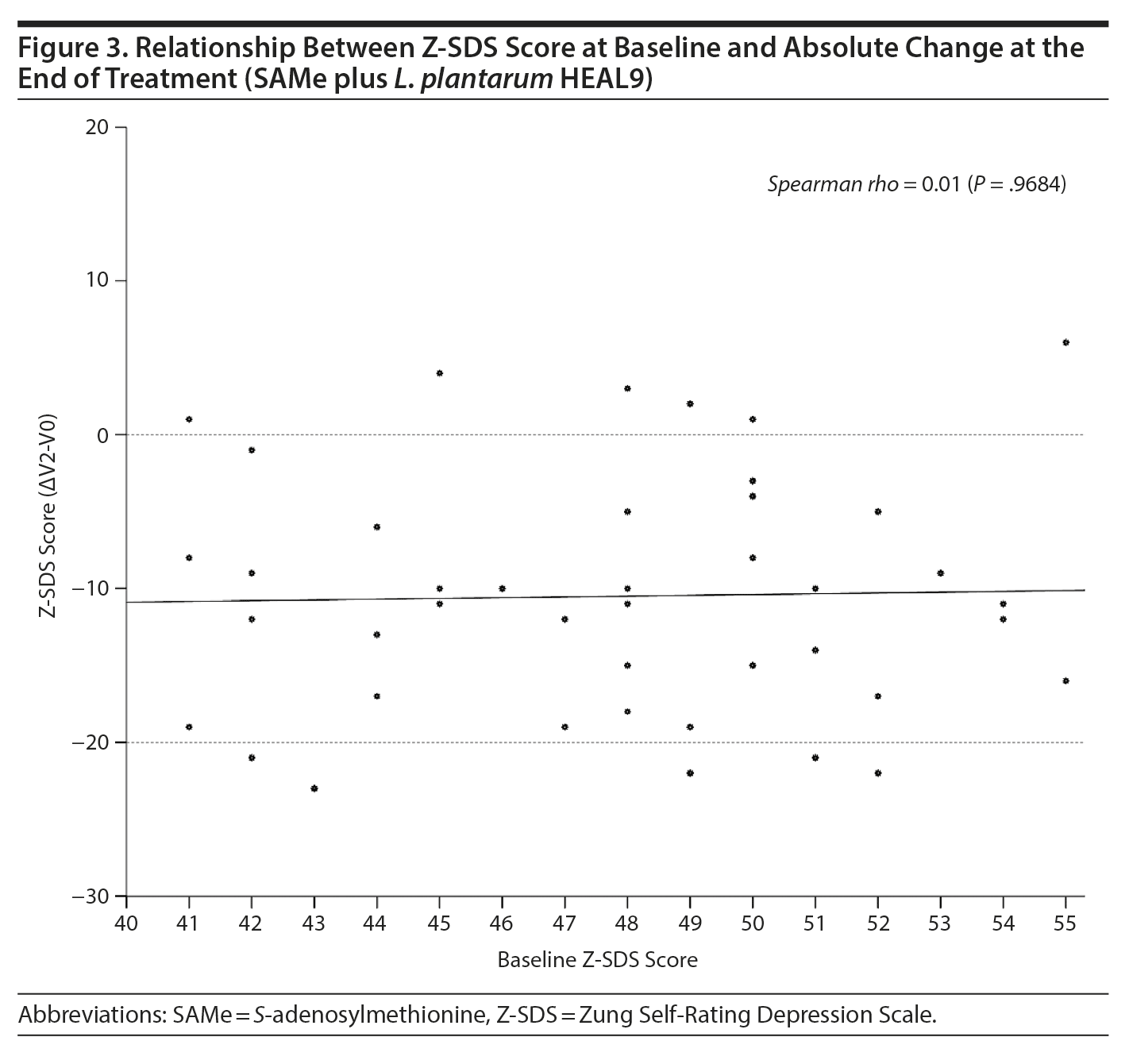
Any relationship between Z-SDS score at baseline and absolute change at the end of treatment for the SAMe plus L. plantarum HEAL9 group was evaluated with Spearman Ï and corresponding P value and then confirmed by graph.
A randomization list was prepared using R statistical software version 3.5. Statistical analyses were performed with SAS version 9.4 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
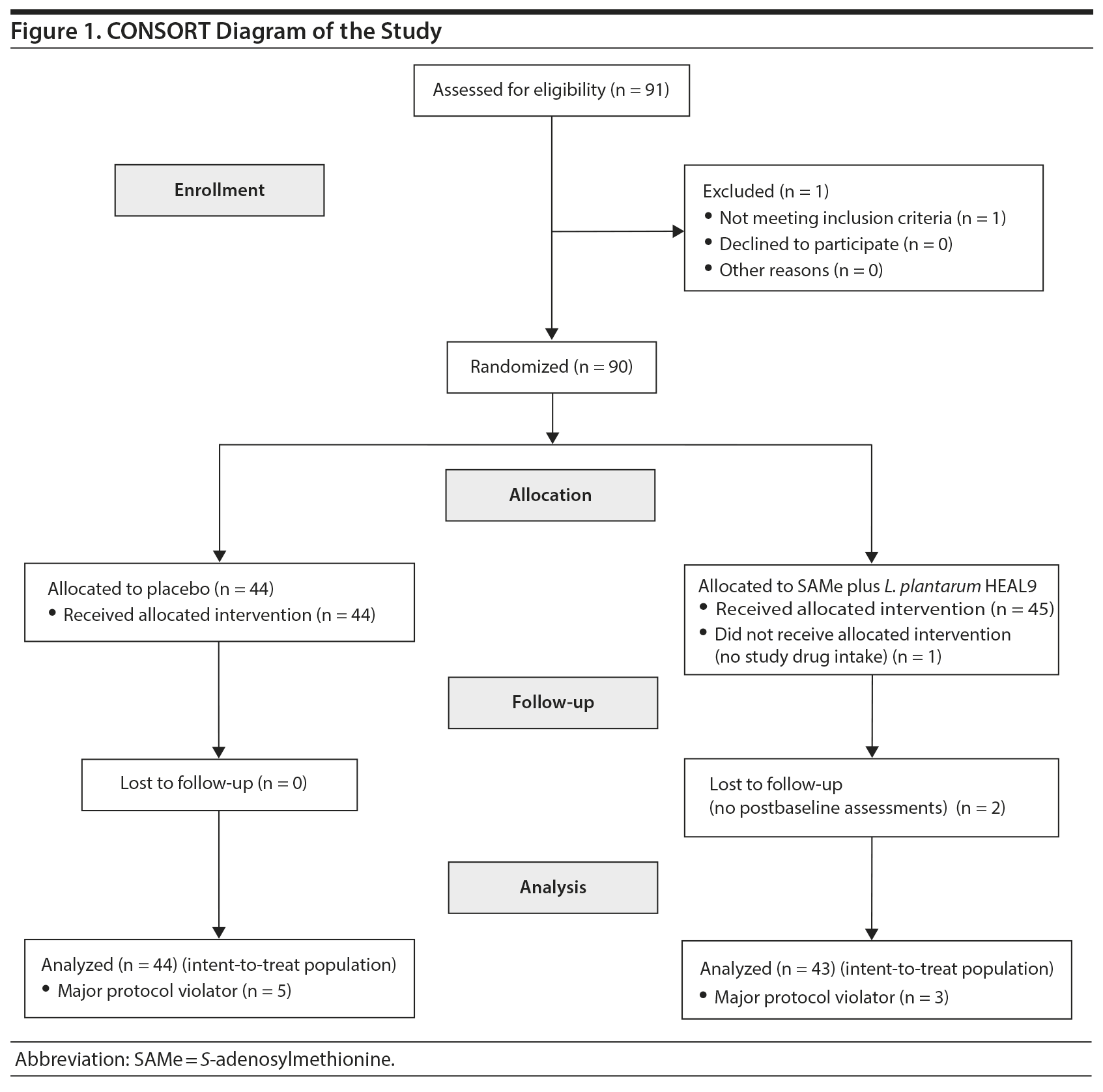
Of 91 screened patients, 90 were randomized to SAMe plus L. plantarum HEAL9 (n = 46) or placebo (n = 44). Eighty-nine (89) subjects were administered at least 1 dose of treatment and therefore were included in the safety population. Two subjects in the study treatment group did not complete postbaseline assessments and thus were excluded from the ITT set, which comprised 87 subjects. Furthermore, a total of 8 major protocol violators (3 in the SAMe plus L. plantarum HEAL9 group and 5 in the placebo group) were not included in the PP set comprised of 79 subjects (Figure 1).
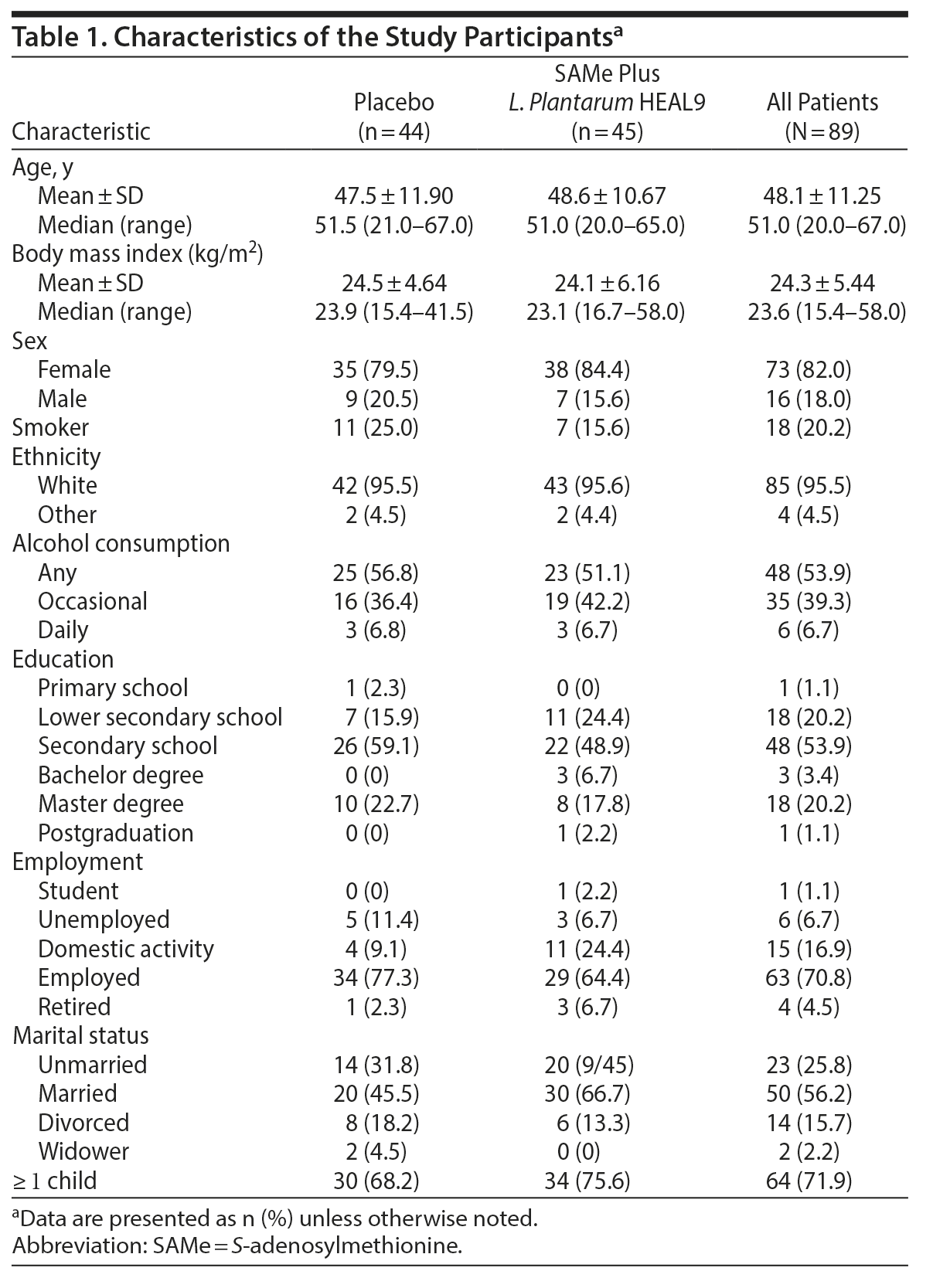
Demographic data, habits, and socioeconomic characteristics are summarized in Table 1. No unblinding occurred. Compliance ≥ 80% was recorded for 95.6% of treated patients.
Efficacy
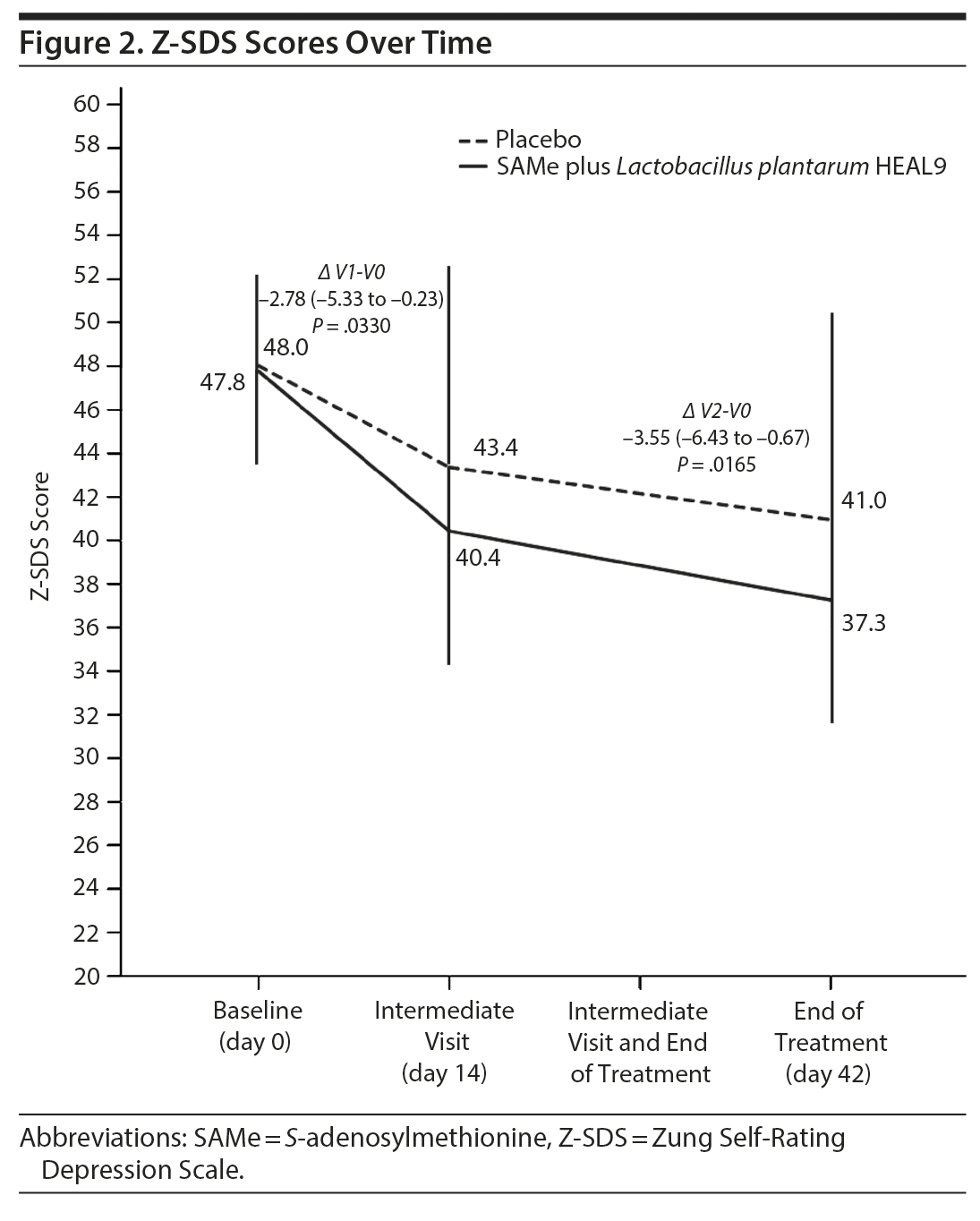
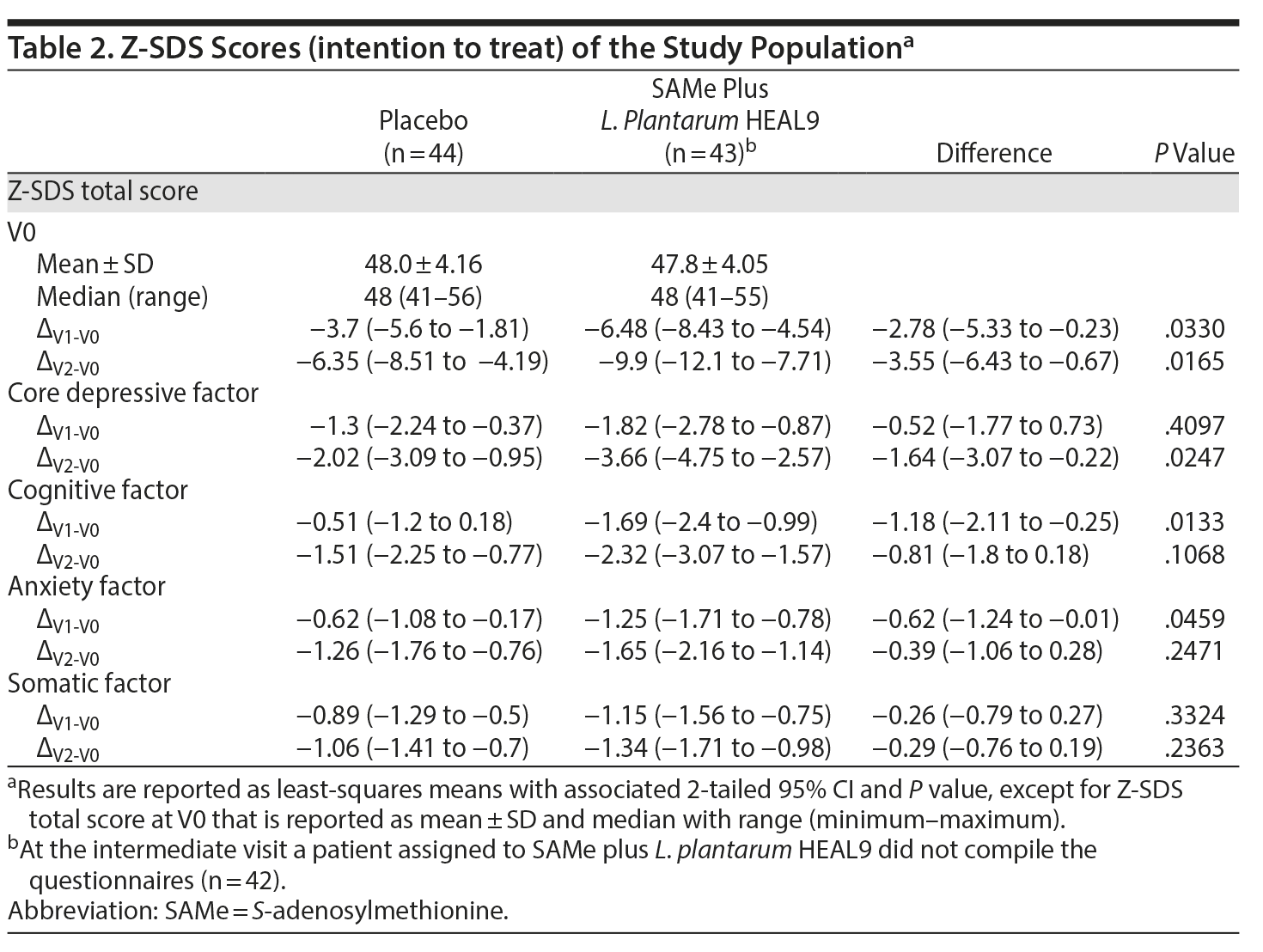
Figure 2 shows the trend over time for the Z-SDS score among the 2 treatment groups. The SAMe plus L. plantarum HEAL9 group had a greater reduction in Z-SDS total score compared to placebo at both treatment week 2 (P = .0330) and treatment week 6 (P = .0165) (Table 2).
With regard to the Z-SDS subdomains, the difference between groups from baseline to the end of treatment was statistically significant (P = .0247) for the core depressive factor, in favor of new treatment. Evaluation of the subgroup of patients treated with SAMe plus L. plantarum HEAL9 showed that the baseline Z-SDS total score did not influence the degree of score reduction observed at the end of treatment (Ï = 0.01, P = .9684) (Figure 3). In addition, after 14 days of treatment, the decrease in scores for both cognitive and anxiety factors was statistically higher for SAMe plus L. plantarum HEAL9 compared to placebo (P = .0133 and P = .0459, respectively) (Table 2).
The analysis of change from baseline to the end of treatment of scores from the other questionnaires showed no statistically significant difference between groups. A higher reduction in favor of the new treatment in the B-IBS total score compared to placebo was noted but did not reach statistical significance (P = .0854). The absolute reduction in Z-SAS total score after 14 days of treatment showed a statistically significant improvement in favor of SAMe plus L. plantarum HEAL9 (P = .0345); however, this result was not confirmed at the end of treatment (P = .3418).
Safety
Adverse events occurred in 1 subject treated with placebo (rash, erythema, and itching) and in 2 subjects treated with the new combination (reduced appetite and depression). These adverse events were regarded as not related to the study products.
DISCUSSION
This randomized, double-blind, placebo-controlled study showed the superior efficacy of a combination of SAMe and L. plantarum HEAL9 compared to placebo and its good safety profile for patients with subthreshold and mild-to-moderate depressive symptoms based on patient-reported outcome measures in a primary care setting.
The superiority of the new product versus placebo is expressed by the statistically significant difference of the mean absolute change of the Z-SDS total score (both ITT and PP sets) from baseline to the end of treatment after 6 weeks (primary endpoint).
A statistically significant difference between groups in absolute change in the Z-SDS total score was evident after 2 weeks as well as in the Z-SDS cognitive and anxiety factors and Z-SAS total score, thus highlighting a rapid onset of action of the new combination treatment.
Several previous studies27-29 assessed the benefits of treatment with SAMe for mild-to-moderate depressive symptoms. The efficacy of SAMe on depressive syndrome has been highlighted since the nineties and has been evaluated in many depression settings. In a double-blind randomized trial,27 the addition of SAMe to an SRI provided a significant improvement and good safety profile compared to placebo in SRI-resistant patients with major depressive disorder. In a small, open-label, randomized, observational study,28 the 3-month administration of SAMe plus betaine versus amitriptyline in 64 subjects with mild depression (diagnosed according to the Z-SDS) provided promising results. In a review by Carpenter and colleagues,29 the majority of randomized clinical studies showed the superiority of SAMe in monotherapy compared to placebo in subjects with mild-to-moderate depressive symptoms. Moreover, SAMe is among the recommended first- or second-line “natural” treatment alternatives to the conventional antidepressants for mild-to-moderate depressive disorders by the Canadian Network for Mood and Anxiety Treatments 2016.30
The 6-week duration of the present study can be considered appropriate according to a systematic review12of trials that evaluated SAMe compared with placebo or conventional antidepressants (mean of 5.3 weeks).
The small number of adverse events observed in this study (judged unrelated to the treatment) and the high rate of patient compliance highlights the good safety profile of the combined treatment.
In conclusion, supplementation with SAMe and L. plantarum HEAL9 in adults with subthreshold or mild-to-moderate symptoms of depression showed fast and clinically relevant effects after 2 weeks of intake. The combination was proved to be safe and showed significant effects on symptoms of depression, including anxiety and cognitive and somatic components. This novel therapeutic combination appears to have effects independent from the severity of symptoms and is an alternative to the traditional antidepressants available on the market today.
Since both TCAs and SSRIs are characterized by either slow onset of action31 or efficacy that is dependent on the baseline severity of symptoms,9 it might be speculated that the action of the combination of SAMe and L. plantarum HEAL9 on depressive symptoms involves mechanisms of action somehow different from those of TCAs and SSRIs.
Limitations of the present study include the relatively small number of subjects and the limited time period of 6 weeks of treatment, hence no long-term conclusions can be drawn. Also, the Z-SDS was used without including professional psychiatric evaluation. Thus far, this is the only study to investigate the effects of SAMe combined with L. plantarum HEAL9, and no results of dose-dependent effects or any potential interactions with concomitant antidepressant pharmacologic treatments have been reported.
Randomized controlled studies with prolonged follow-up investigating the effect of this combination in more severe forms of depression or in combination with antidepressants are necessary to determine its potential and any benefits in the treatment of depression.
Submitted: December 5, 2019; accepted February 19, 2020.
Published online: June 25, 2020.
Author contributions: Drs Saccarello, Montarsolo, Massardo, Picciotto, Pedemonte, Castagnaro, Brasesco, Guida, and Picco conducted the study and collected clinical data. Dr Fioravanti contributed to the study design, data interpretation and analysis, and critical revision. Dr Schiavetti performed the literature search and critical revision and wrote the manuscript. Drs Montisci and Vanelli were involved in the critical revision of the manuscript. All authors had full responsibility for the content of this article.
Potential conflicts of interest: Drs Saccarello, Montarsolo, Massardo, Picciotto, Pedemonte, Castagnaro, Brasesco, Guida, and Picco received research support for this study from Nutrilinea SRL. Drs Fioravanti, Montisci, and Schiavetti are, respectively, the medical director, clinical project manager, and clinical data manager of Hippocrates Research SRL, the clinical research organization in charge of the study that received fees for this service. Dr Vanelli is an employee of Nutrilinea SRL, which provided financial and material support for the design and concept of the study, data collection, data management, data analysis, and publication of this study.
Funding/support: This study was supported by Nutrilinea SRL, Varese, Italy.
Role of the sponsor: The funder of the study supplied materials and had a role in reviewing and approving the study design, data collection, data management, data analysis, and publication of this article.
Acknowledgments: The authors acknowledge the excellent assistance of Nutrilinea SRL and the staff of Hippocrates Research SRL. In particular, Giulia Rosso, MS, clinical trial monitor of Hippocrates Research, which supported general practitioners in collecting clinical data, is gratefully acknowledged. Ms Rosso reports no other conflicts of interest related to the subject of this article.
REFERENCES
1.Depression and Other Common Mental Disorders: Global Health Estimates. World Health Organization; website. https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf. 2017.
2.The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992.
3.Machaczek KK, Allmark P, Goyder E, et al. A scoping study of interventions to increase the uptake of physical activity (PA) amongst individuals with mild-to-moderate depression (MMD). BMC Public Health. 2018;18(1):392. PubMed CrossRef
4.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
5.Pincus HA, Davis WW, McQueen LE. ‘ Subthreshold’ mental disorders. A review and synthesis of studies on minor depression and other ‘ brand names’ . Br J Psychiatry. 1999;174(4):288-296. PubMed CrossRef
6.Kuboki T, Hashizume M. Clinical diagnosis and treatment of mild depression. JMAJ. 2011;54(2):76-80.
7.Hyde J, Calnan M, Prior L, et al. A qualitative study exploring how GPs decide to prescribe antidepressants. Br J Gen Pract. 2005;55(519):755-762. PubMed
8.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53. PubMed CrossRef
9.Mart×nez-Cengotitabengoa M, González-Pinto A. Nutritional supplements in depressive disorders. Actas Esp Psiquiatr. 2017;45(suppl):8-15. PubMed
10. Mischoulon D, Alpert JE, Arning E, et al. Bioavailability of S-adenosyl methionine and impact on response in a randomized controlled study in major depressive disorder. J Clin Psychiatry. 2012;73(6):843-848. PubMed CrossRef
11. Galizia I, Oldani L, Macritchie K, et al. S-adenosyl methionine (SAMe) for depression in adults. Cochrane Database Syst Rev. 2016;10:CD011286. PubMed
12. Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry. 2017;16(1):14. PubMed CrossRef
13. Huang R, Wang K, Hu J. Effects of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):483. PubMed CrossRef
14. Rudzki L, Ostrowska L, Pawlak D, et al. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213-222. PubMed CrossRef
15. Andersson H, Tullberg C, Ahrné S, et al. Oral administration of Lactobacillus plantarum 299v reduces cortisol levels in human saliva during examination induced stress: a randomized, double-blind controlled trial. Int J Microbiol. 2016;2016:8469018. PubMed CrossRef
16. Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13(10):1143-1147. PubMed CrossRef
17. Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18(30):4012-4018. PubMed CrossRef
18. Nobaek S, Johansson ML, Molin G, et al. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95(5):1231-1238. PubMed CrossRef
19. Zung WWK. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371-379. PubMed CrossRef
20. Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26(6):701-710. PubMed CrossRef
21. Roalfe AK, Roberts LM, Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8(1):30. PubMed CrossRef
22. EQ-5D-3L Instruments. Version 17. EQ-5D website. https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/. April 2017. Accessed May 28, 2020.
23. EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. PubMed CrossRef
24. Dunstan DA, Scott N, Todd AK. Screening for anxiety and depression: reassessing the utility of the Zung scales. BMC Psychiatry. 2017;17(1):329. PubMed CrossRef
25. Zung WWK. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70. PubMed CrossRef
26. de Jonghe JF, Baneke JJ. The Zung Self-Rating Depression Scale: a replication study on reliability, validity and prediction. Psychol Rep. 1989;64(3):833-834. CrossRef
27. Papakostas GI, Mischoulon D, Shyu I, et al. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167(8):942-948. PubMed CrossRef
28. Di Pierro F, Settembre R. Preliminary results of a randomized controlled trial carried out with a fixed combination of S-adenosyl-L-methionine and betaine versus amitriptyline in patients with mild depression. Int J Gen Med. 2015;8:73-78. PubMed
29. Carpenter DJ. St John’s wort and S-adenosylmethionine as “natural” alternatives to conventional antidepressants in the era of the suicidality boxed warning: what is the evidence for clinically relevant benefit? Altern Med Rev. 2011;16(1):17-39. PubMed
30. Ravindran AV, Balneaves LG, Faulkner G, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder, section 5: complementary and alternative medicine treatments. Can J Psychiatry. 2016;61(9):576-587. PubMed CrossRef
31. Machado-Vieira R, Baumann J, Wheeler-Castillo C, et al. The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals (Basel). 2010;3(1):19-41. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top