Prim Care Companion CNS Disord 2022;24(2):21cr02976
To cite: Bowers P, Rosenkrantz B, Palanci J, et al. A slow, cautious, and successful clozapine rechallenge after myocarditis. Prim Care Companion CNS Disord. 2022;24(2):21cr02976.
To share: https://doi.org/10.4088/PCC.21cr02976
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Behavioral Sciences, Emory University, Atlanta, Georgia
*Corresponding author: Philip Bowers, MD, 12 Executive Park Dr NE #200, Atlanta, GA 30329 ([email protected]).
United States guidelines recommend clozapine for the treatment of schizophrenia when an individual has inadequate response to 2 adequately dosed antipsychotic trials.1 Despite superior efficacy,2,3 prescriber concern for life-threatening effects is one of the main drivers for its underutilization.4 Clozapine-induced myocarditis (CIM) is one such life-threatening event, occurring in 0.7% of people exposed to clozapine, most commonly within the first month of clozapine titration.5,6 Notably, incidence appears to be higher in Australia (2%) compared to the rest of the world (0.3%), possibly due to increased echocardiographic screening in Australia after the year 2000.6,7 Myocarditis screening protocols, which commonly include close monitoring for clinical signs and symptoms, vital sign monitoring, and laboratory tests including C-reactive protein (CRP) and troponin I/T, may facilitate the early identification of clozapine-induced myocarditis.8 However, if an individual develops CIM, clinicians face the dilemma of whether to rechallenge with clozapine, especially since many individuals have not adequately responded to other antipsychotic medications and are actively experiencing impairing symptoms. We present a case of an otherwise healthy 23-year-old man with schizophrenia who was successfully rechallenged with clozapine after developing myocarditis.
Case Report
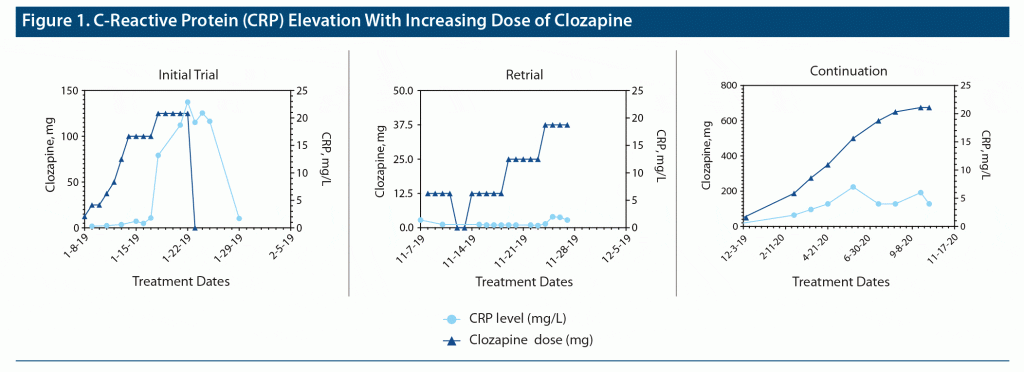
The patient is a 23-year-old, single, Latin American man with no personal or family history of psychiatric or medical illness, who was diagnosed at age 20 years with schizophrenia. His primary symptoms included prominent persecutory beliefs, command auditory hallucinations to self-harm, and suicidal ideation. After several month trials of risperidone 8 mg/d, which was ineffective and resulted in hyperprolactinemia, and olanzapine 30 mg/d with only modest response, a trial of clozapine was initiated. Clozapine was titrated gradually to 125 mg over a 10-day period (Figure 1). On day 16, he developed tachycardia to the 120s and diaphoresis, and electrocardiography (EKG) reported anterolateral Q waves. His troponin level was acutely elevated to 0.68 ng/mL, and his CRP was elevated to 22.9 mg/L (normal is < 3 mg/L). Serum clozapine/norclozapine levels were not obtained, though immediate discontinuation of clozapine resulted in symptomatic improvement and laboratory normalization. A transthoracic echocardiogram (TTE) was grossly normal with an ejection fraction of 60%, ruling out possible diagnosis of pericarditis. Cardiology was consulted and made a preliminary diagnosis of CIM given the following: timeline of symptom development, lack of apparent infectious etiology, elevation in cardiac biomarkers, known risk of cardiotoxicity with clozapine, and a lack of additional cardiac risk factors. The patient’s body mass index was 26.81 kg/m2 and cholesterol levels were normal, arguing against atherosclerotic origin of the symptoms. Cardiac magnetic resonance imaging was recommended for confirmation; however, this was not obtained as the patient did not attend his imaging appointment. He was restarted on olanzapine given previous modest response, which was titrated back to 30 mg/d.
Ten months after clozapine discontinuation, the patient was hospitalized for worsening self-harm in response to command auditory hallucinations. A new EKG showed diffuse Q-waves consistent with a history of myocarditis. The patient, his family, and his outpatient psychiatrist collaboratively decided to reinitiate clozapine. Clozapine was restarted at 12.5 mg nightly and titrated to 12.5 mg weekly, with concurrent prescription of olanzapine 30 mg/d for ongoing psychotic symptoms. Troponin and CRP levels were obtained twice weekly for 6 months, at which point the daily clozapine dose was 325 mg. In the absence of troponin or CRP elevation, there was some mild persistent tachycardia to a rate of 111 beats/minute, which normalized to an average rate of 95 beats/minute after introducing metoprolol succinate 25 mg/d. Within a year after rechallenge, clozapine was titrated to 600 mg nightly, olanzapine was discontinued, and the patient’s auditory hallucinations and self-harm urges significantly improved without recurrence of diaphoresis or cardiac complaints.
Discussion
To our knowledge, this is the 18th reported successful clozapine rechallenge after CIM out of now 26 retrials and 1 dose continuation in the literature.9–16 Our patient’s TTE was grossly normal, though a third of cases per the sample of Ronaldson et al5 did not have left ventricular impairment seen on echocardiogram. With careful monitoring, rechallenge after CIM can be considered: (1) in patients with limited response to other antipsychotics, (2) in patients with ongoing cardiology collaboration, (3) using a slow titration schedule (eg, 12.5 mg/wk increases), and (4) utilizing a screening protocol that includes routine monitoring of troponin and CRP at a minimum. Considered in the context of cardiovascular symptoms and troponin level, fluctuations in CRP may be nonspecific to CIM and could occur due to other causes including smoking, stress, infection, or increased weight.17 This patient lacked common risk factors for CIM, such as an initial rapid titration and concurrent use of sodium valproate.18 The absence of these factors possibly contributed to a successful retitration. Further research is needed to guide clinicians about the optimum protocol for rechallenge after CIM. Given a post-myocarditis retrial success rate of nearly two-thirds, post-CIM rechallenge may be feasible with close monitoring, cardiology collaboration, and a detailed informed consent process.
Published online: March 10, 2022.
Potential conflicts of interest: Dr Cotes has received research funding from Otsuka, Lundbeck, Roche, and Alkermes and was a consultant to Saladax Biomedical. Drs Bowers, Rosenkrantz, Palanci, and Goldsmith report no conflicts of interest related to the subject of this report.
Funding/support: None.
Patient consent: Verbal consent was received from the patient to publish this case report, and information has been de-identified to protect anonymity.
References (18)

- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. Third Edition. APA website. Accessed January 15, 2021. https://www.psychiatry.org/psychiatrists/practice/clinical-practice-guidelines
- Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31–41. PubMed CrossRef
- Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385–392. PubMed CrossRef
- Verdoux H, Quiles C, Bachmann CJ, et al. Prescriber and institutional barriers and facilitators of clozapine use: a systematic review. Schizophr Res. 2018;201:10–19. PubMed CrossRef
- Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458–465. PubMed CrossRef
- Siskind D, Sidhu A, Cross J, et al. Systematic review and meta-analysis of rates of clozapine-associated myocarditis and cardiomyopathy. Aust N Z J Psychiatry. 2020;54(5):467–481. PubMed CrossRef
- Dawson JL, Clark SR, Sluggett JK, et al. Is the higher incidence of clozapine induced myocarditis in Australia due to awareness and monitoring? Schizophr Res. 2018;201:426–427. PubMed CrossRef
- Goldsmith DR, Cotes RO. An unmet need: a clozapine-induced myocarditis screening protocol. Prim Care Companion CNS Disord. 2017;19(4):16l02083. PubMed CrossRef
- Danilewitz M, Rafizadeh R, Bousman CA. Successful clozapine rechallenge after suspected clozapine-associated myocarditis: a case report. J Clin Psychopharmacol. 2021;41(2):218–220. PubMed CrossRef
- Higgins JM, San C, Lagnado G, et al. Incidence and management of clozapine-induced myocarditis in a large tertiary hospital. Can J Psychiatry. 2019;64(8):561–567. PubMed CrossRef
- Hosseini SA, Skrzypcak B, Yasaei R, et al. Successful clozapine re-challenge after suspected clozapine-induced myocarditis. Am J Case Rep. 2020;21:e926507. PubMed CrossRef
- Manu P, Lapitskaya Y, Shaikh A, et al. Clozapine rechallenge after major adverse effects: clinical guidelines based on 259 cases. Am J Ther. 2018;25(2):e218–e223. PubMed CrossRef
- Noël MC, Powell V, Burton L, et al. Clozapine-related myocarditis and rechallenge: a case series and clinical review. J Clin Psychopharmacol. 2019;39(4):380–385. PubMed CrossRef
- Shivakumar G, Thomas N, Sollychin M, et al. Protocol for clozapine rechallenge in a case of clozapine-induced myocarditis. Can J Psychiatry. 2020;65(7):448–453. PubMed CrossRef
- Whiskey E, Yuen S, Khosla E, et al. Resolution without discontinuation: heart failure during clozapine treatment. Ther Adv Psychopharmacol. 2020;10:2045125320924786. PubMed CrossRef
- Griffin JM, Woznica E, Gilotra NA, et al. Clozapine-associated myocarditis: a protocol for monitoring upon clozapine initiation and recommendations for how to conduct a clozapine rechallenge. J Clin Psychopharmacol. 2021;41(2):180–185. PubMed CrossRef
- Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. PubMed CrossRef
- Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Rapid clozapine dose titration and concomitant sodium valproate increase the risk of myocarditis with clozapine: a case-control study. Schizophr Res. 2012;141(2-3):173–178. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top