Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are acute life-threatening conditions that present with skin erosions and extensive detachment of the epidermis that is typically caused by drug exposure within weeks to months. Although rare conditions, they are potentially lethal even among healthy patients. Among anticonvulsants, phenytoin, lamotrigine, and carbamazepine are most often associated with SJS/TEN, while valproic acid is viewed as low risk.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are acute life-threatening conditions that present with skin erosions and extensive detachment of the epidermis that is typically caused by drug exposure within weeks to months. Although rare conditions, they are potentially lethal even among healthy patients. Among anticonvulsants, phenytoin, lamotrigine, and carbamazepine are most often associated with SJS/TEN,1-3 while valproic acid is viewed as low risk. However, the risk of valproic acid with regard to SJS/TEN may be underestimated in current clinical practice.
Case Report
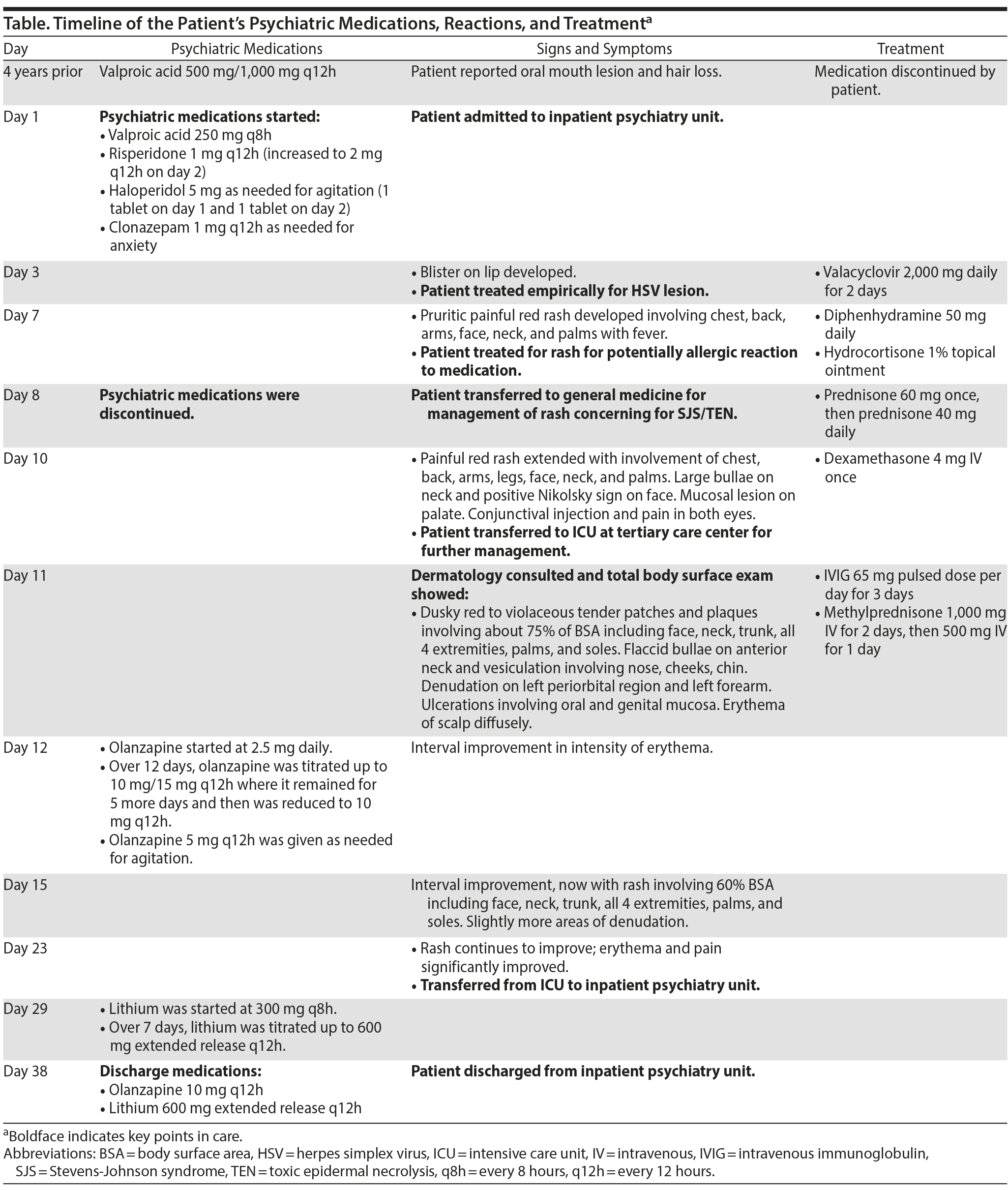
A patient was admitted to the inpatient psychiatry unit of an outside hospital for management of bipolar I disorder and prescribed haloperidol, risperidone, valproic acid, and benztropine. Several days after treatment began, the patient developed a blister on lip mucosa, diffuse rash, and dysphagia, which is concerning for possible SJS. Psychiatric medications were held, and the patient was transferred to the medical intensive care unit of a tertiary care center. An intact, diffuse rash involving 75% of body surface area and epidermal detachment of 1%-2% of body surface area was found, which is consistent with SJS. Pathology of a skin biopsy showed necrotic epidermis with epidermolysis and confirmed the diagnosis of SJS/TEN. A 3-day course of intravenous immunoglobulin and tapered pulse methylprednisolone was started. Psychiatric symptoms were managed with olanzapine, lithium, and clonazepam. The Table includes a timeline of the patient’s psychiatric medications, reactions, and treatment.
The patient reported 1 prior reaction after receiving valproic acid 500 mg/1,000 mg every 12 hours while admitted to an inpatient psychiatry unit 4 years prior (hair loss and an oral blister), resulting in self-discontinuation of valproic acid and resolution of symptoms. She never continued valproic acid or other psychiatric medications as an outpatient. Using the Algorithm of Drug Causality for Epidermal Necrolysis4 and given her prior reaction, we determined that valproic acid was a very probable cause of the patient’s SJS/TEN, in contrast to haloperidol, risperidone, and benztropine, which were determined very unlikely causes.
Conclusion
Further research is needed to define the risk for SJS/TEN with valproic acid when used in the absence of other anticonvulsants. The risk for SJS/TEN with valproic acid monotherapy may be underestimated in clinical practice. Furthermore, valproic acid and lamotrigine in combination have increased risk for SJS/TEN,2,3,6 and it is possible that other combinations do as well. Physician knowledge regarding risk can guide clinical decision-making and facilitate early diagnosis and treatment of SJS/TEN.
Lastly, the risk of anticonvulsant-induced SJS/TEN has been associated with genetic and ethnic background. For example, the HLA-B*1502 genotype has a 100% sensitivity and 97% specificity for carbamazepine-induced SJS/TEN in a Han Chinese population.11 Additional epidemiology and genetic studies are needed. It may be possible to reduce SJS/TEN by avoiding specific anticonvulsants in high-risk ethnic backgrounds or by screening for high-risk genetic variants. Genetic screening has already been successfully implemented in 1 large Taiwanese study,12 which successfully avoided carbamazepine-induced SJS/TEN in patients with the HLA-B*1502 allele.
Potential conflicts of interest: None.
Funding/support: None.
Additional information: Information has been de-identified to protect anonymity.
Published online: March 28, 2019.
aDivision of Child Neurology, Weill Cornell Medicine, New York, New York
bDepartment of Psychiatry, Weill Cornell Medicine, New York, New York
*Corresponding author: Caitlin E. Snow, MD, Department of Psychiatry, Weill Cornell Medicine, 407 E 61st St, New York, New York 10065 ([email protected]).
Prim Care Companion CNS Disord 2019;21(2):18l02374
To cite: Barbour KK, Umfrid CP, Brinton BA, et al. A case of Stevens-Johnson syndrome after exposure to valproic acid. Prim Care Companion CNS Disord. 2019;21(2):18l02374.
To share: https://doi.org/10.4088/PCC.18l02374
© Copyright 2019 Physicians Postgraduate Press, Inc.
References
1. Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333(24):1600-1607. PubMed CrossRef
2. Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128(1):35-44. PubMed CrossRef
3. Ordo×±ez L, Salgueiro E, Jimeno FJ, et al. Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs. Eur Rev Med Pharmacol Sci. 2015;19(14):2732-2737. PubMed
4. Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88(1):60-68. PubMed CrossRef
5. Tennis P, Stern RS. Risk of serious cutaneous disorders after initiation of use of phenytoin, carbamazepine, or sodium valproate: a record linkage study. Neurology. 1997;49(2):542-546. PubMed CrossRef
6. Schmidt D, Schachter SC. Drug treatment of epilepsy in adults. BMJ. 2014;348:g254. PubMed CrossRef
7. Misra BN, Mohapatra PK, Roy D. Stevens-Johnson syndrome during treatment with lithium and valproate in mood disorder: a report of two cases. Indian J Psychiatry. 2002;44(3):301-302. PubMed
8. Naveen K, Arunkumar J, Hanumanthayya K, et al. Stevens-Johnson syndrome induced by sodium valproate monotherapy. Int J Crit Illn Inj Sci. 2012;2(1):44-45. PubMed CrossRef
9. Kumar PN, Kumar SK. Stevens-Johnson syndrome induced by sodium valproate. Indian J Psychiatry. 2004;46(3):269-270. PubMed
10. Saaiq M, Iqbal T. Report: fatal toxic epidermal necrolysis induced by sodium valproate. Pak J Pharm Sci. 2013;26(3):637-639. PubMed
11. Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428(6982):486. PubMed CrossRef
12. Chen P, Lin JJ, Lu CS, et al; Taiwan SJS Consortium. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364(12):1126-1133. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top