Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Approximately 17%-80% of people with schizophrenia use cannabis, and one-quarter have a lifetime cannabis use disorder. Withdrawal symptoms are clinically significant because they may act as negative reinforcement for relapse to cannabis use. We previously published a cross-sectional survey on the experience of cannabis withdrawal (assessed with the Marijuana Quit Questionnaire [MJQQ]) in 120 adults with schizophrenia who made a "serious" (self-defined) quit attempt with no formal treatment while not in a controlled environment (index quit attempt).
Psychoactive Substance Use by Adults With Schizophrenia Before and During Cannabis Withdrawal
To the Editor: Approximately 17%-80% of people with schizophrenia use cannabis,1-3 and one-quarter have a lifetime cannabis use disorder.4 Withdrawal symptoms are clinically significant because they may act as negative reinforcement for relapse to cannabis use.5,6 We previously published a cross-sectional survey7,8 on the experience of cannabis withdrawal (assessed with the Marijuana Quit Questionnaire [MJQQ]) in 120 adults with schizophrenia who made a "serious" (self-defined) quit attempt with no formal treatment while not in a controlled environment (index quit attempt). Here, we extend those findings by presenting data on psychoactive substance use before and during the index quit attempt among the same cohort.
Methods. Participants were a convenience sample of adults (aged 18 years or older) with a chart diagnosis of schizophrenia or schizoaffective disorder (DSM-IV criteria) recruited from community outpatient mental health treatment programs in the Baltimore, Maryland, metropolitan area (December 2006-July 2011) who used cannabis at least weekly for 6 months prior to the index quit attempt. Data were collected using the MJQQ,6 an individually administered, 176-item, semistructured, self-report questionnaire that collects information on sociodemographic data and cannabis use history, and index quit attempt characteristics, including changes in other substance use. Participants had to show ability to give valid informed consent on the basis of the evaluation to sign consent process.9 The Institutional Review Boards of the University of Maryland, Baltimore; the Maryland Department of Health and Mental Hygiene; the Sheppard Pratt Health System, Baltimore, Maryland; and the National Institute on Drug Abuse Intramural Research Program approved the study (ClinicalTrials.gov identifier: NCT00679016). The study and procedures were fully explained, and written informed consent was obtained from all participants, who were paid for their participation. Descriptive statistics are reported as number (percentage) for categorical data and mean (range) for age.
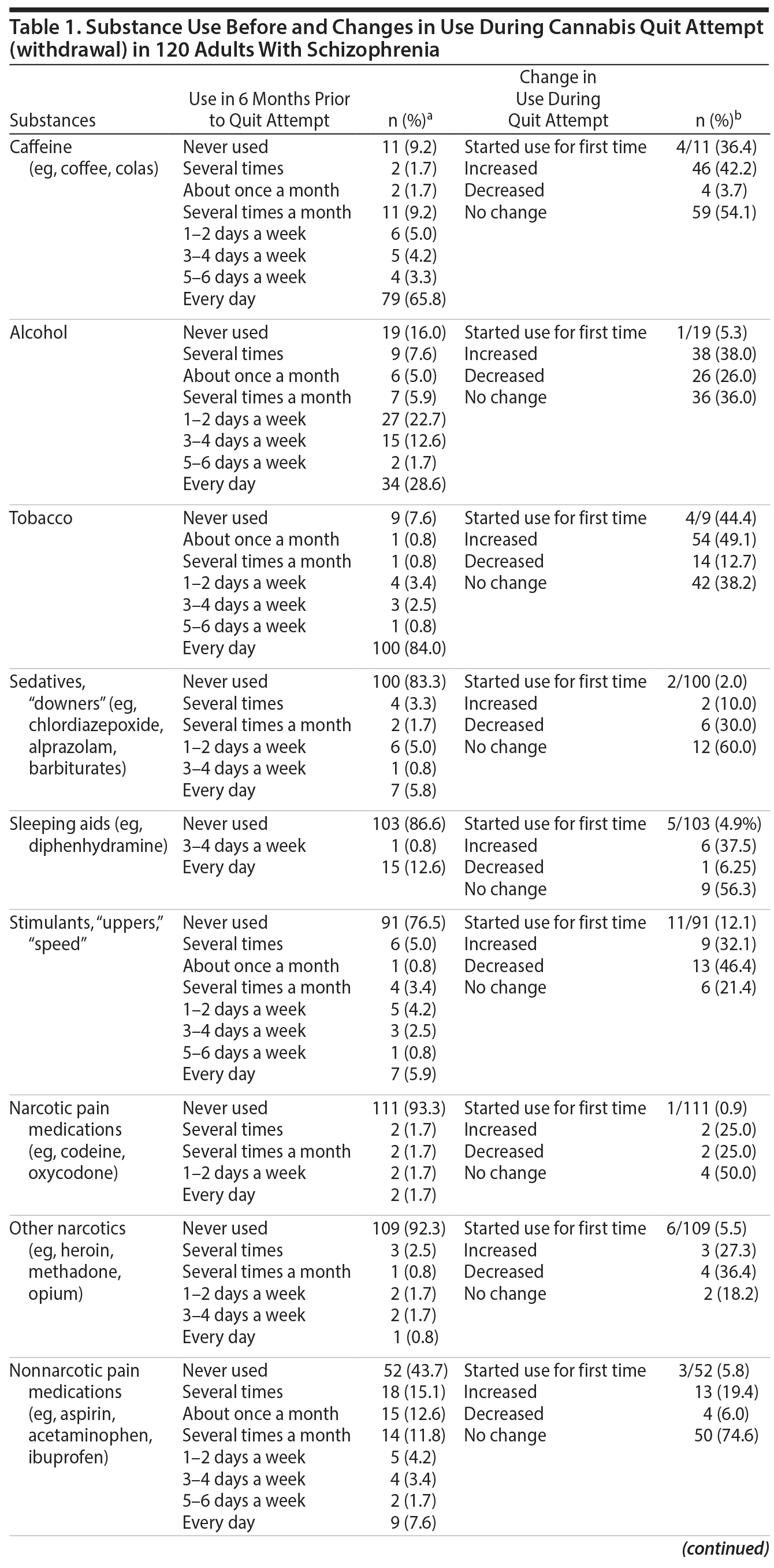
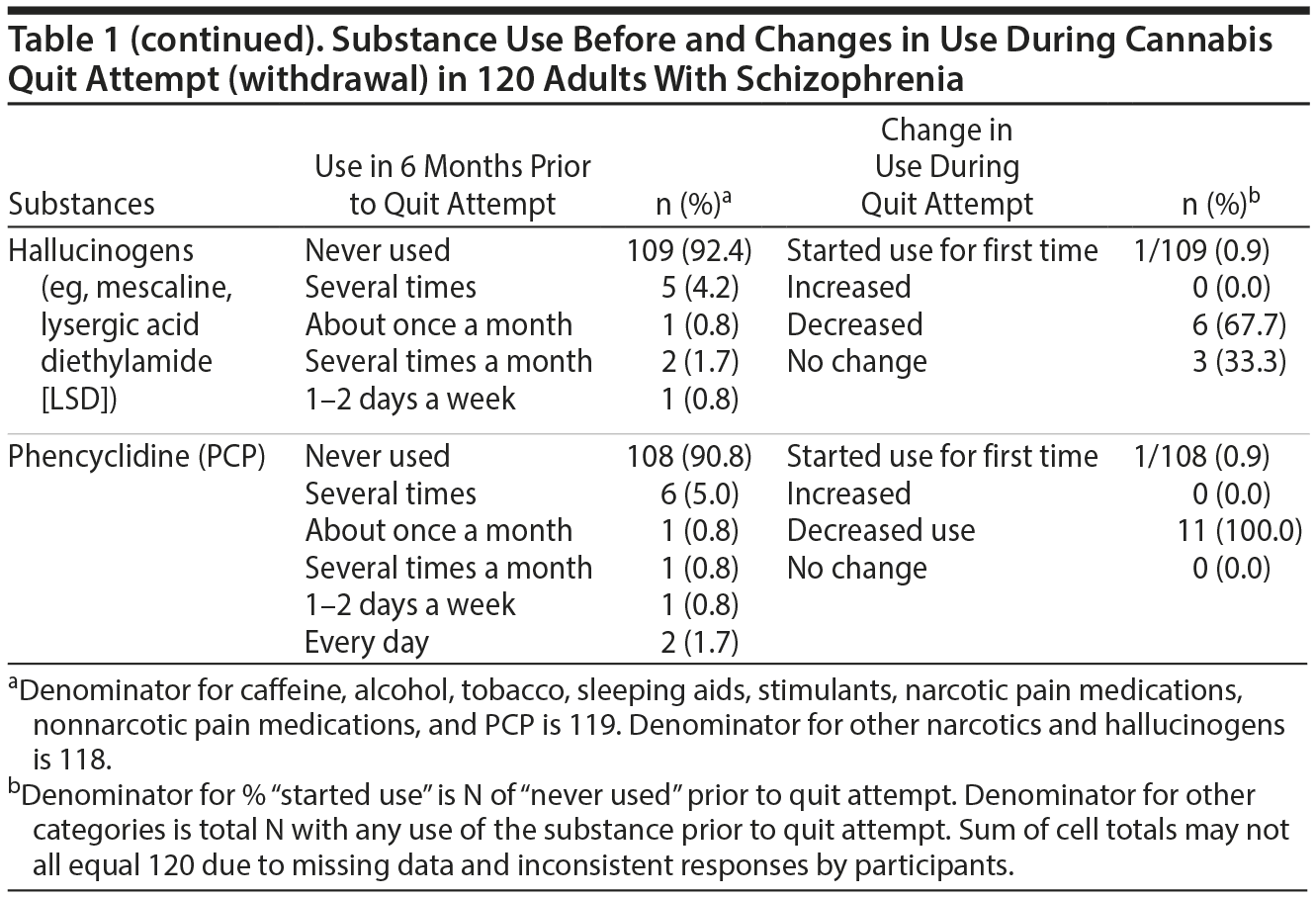
Results. A full description of participants was previously published.7 Briefly, three-quarters were men and 62.5% were black. The mean (range) age at the time of interview was 41.5 (21.3-63.3) years; age at start of index quit attempt was 29.3 (15.4-59.1) years. The mean (range) interval between start of the index quit attempt and the interview was 9 years (1 day-37 years). Among the 76 (63.3%) participants who had resumed cannabis use by the time of the interview, the median (range) duration of abstinence was 182 days (1 day-10 years). Frequency of substance use during the 6 months prior to the quit attempt and changes in use during the quit attempt (cannabis withdrawal) are summarized in Table 1. During quit attempts, participants substantially increased preexisting levels of use of several psychoactive substances (caffeine, alcohol, and tobacco), perhaps to self-medicate cannabis withdrawal symptoms. Initiation of use was uncommon, except for caffeine and tobacco. The proportion of subjects initiating or increasing caffeine, alcohol, or tobacco use is roughly comparable to that found in a study6 using the MJQQ in 469 adult cannabis smokers with no serious psychiatric comorbidity.
This study has several strengths, including the large sample size (N = 120) and detailed substance use histories. The study is limited because the data were collected by retrospective self-report (with no external or objective corroboration) at widely varying lengths of time after the index quit attempt from a convenience sample at a single site. The interval between start of the index quit attempt and the interview was 1 day-37 years. The duration of abstinence at time of interview was 1 day-10 years. These broad ranges suggest that recall bias could have influenced the study results. However, there is evidence that cannabis users give reliable retrospective self-report about their cannabis withdrawal symptoms.10 This study did not collect clinical information about schizophrenia before or during the index quit attempt.
Cannabis withdrawal is a major public health problem leading to relapse of cannabis use. Understanding cannabis withdrawal and associated substance use is critical and timely because cannabis withdrawal is a diagnosis newly added in the DSM-5. Because there are no approved pharmacologic treatments for cannabis withdrawal, there is a clinically unmet need for improved psychosocial treatment interventions focused on psychoactive substance use. Withdrawal symptoms are clinically significant because they may act as negative reinforcement for substance relapse. Smoking cessation programs should be recommended to patients due to the increased use of tobacco during cannabis withdrawal.
References
1. Green B, Young R, Kavanagh D. Cannabis use and misuse prevalence among people with psychosis. Br J Psychiatry. 2005;187(4):306-313. PubMed doi:10.1192/bjp.187.4.306
2. Barnett JH, Werners U, Secher SM, et al. Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry. 2007;190(6):515-520. PubMed doi:10.1192/bjp.bp.106.024448
3. Koola MM, McMahon RP, Wehring HJ, et al. Alcohol and cannabis use and mortality in people with schizophrenia and related psychotic disorders. J Psychiatr Res. 2012;46(8):987-993. PubMed doi:10.1016/j.jpsychires.2012.04.019
4. Koskinen J, Löhönen J, Koponen H, et al. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull. 2010;36(6):1115-1130. PubMed doi:10.1093/schbul/sbp031
5. Copersino ML, Boyd SJ, Tashkin DP, et al. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict. 2006;15(1):8-14. PubMed doi:10.1080/10550490500418997
6. Levin KH, Copersino ML, Heishman SJ, et al. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend. 2010;111(1-2):120-127. PubMed doi:10.1016/j.drugalcdep.2010.04.010
7. Boggs DL, Kelly DL, Liu F, et al. Cannabis withdrawal in chronic cannabis users with schizophrenia. J Psychiatr Res. 2013;47(2):240-245. PubMed doi:10.1016/j.jpsychires.2012.10.010
8. Koola MM, Boggs DL, Kelly DL, et al. Relief of cannabis withdrawal symptoms and cannabis quitting strategies in people with schizophrenia. Psychiatry Res. 2013;209(3):273-278. PubMed doi:10.1016/j.psychres.2013.07.044
9. DeRenzo EG, Conley RR, Love R. Assessment of capacity to give consent to research participation: state-of-the-art and beyond. J Health Care Law Policy. 1998;1(1):66-87. PubMed
10. Mennes CE, Ben Abdallah A, Cottler LB. The reliability of self-reported cannabis abuse, dependence and withdrawal symptoms: multisite study of differences between general population and treatment groups. Addict Behav. 2009;34(2):223-226. PubMed doi:10.1016/j.addbeh.2008.10.003
aClinical Research Program, Sheppard Pratt Health System, Baltimore, Maryland, and Department of Psychiatry and Behavioral Sciences, George Washington University School of Medicine and Health Sciences, Washington, DC
bMaryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine, Baltimore
cVeterans Affairs Connecticut Healthcare System, Department of Psychiatry, Yale University School of Medicine, New Haven
Author contributions: Drs Kelly, Boggs, and Gorelick designed the study and developed the protocol. Dr McMahon and Ms Liu performed the statistical analyses. Dr Koola wrote the first draft of the manuscript, with contributions from Dr Gorelick. All authors approved the final manuscript.
Potential conflicts of interest: Dr Kelly served on the advisory boards for XOMA and Lundbeck. Dr McMahon has been a statistical consultant for Amgen Inc. Drs Koola, Boggs, and Gorelick and Ms Liu report no conflicts of interest related to the subject of this letter.
Funding/support: This study was supported by the Intramural Research Program, National Institute on Drug Abuse (NIDA), National Institutes of Health, and NIDA Residential Research Support Services Contract HHSN271200599091CADB (N01DA-5-9909, Dr Kelly, principal investigator), with additional support from National Institute of Mental Health (NIMH) grant R03 MH076985-01 (Dr Kelly, principal investigator). Manuscript preparation was supported by NIMH T32 grant MH067533-07 and the American Psychiatric Association/Kempf Fund Award for Research Development in Psychobiological Psychiatry (Dr Koola).
Role of the sponsor: The funders played no role in the design, conduct, or analysis of the study; preparation of the manuscript; or the decision to submit for publication.
Acknowledgments: The authors thank Ann Marie Kearns, MS, for help with regulatory compliance; Stephanie Feldman, MSW, LCSW-C, for study supervision and staff coordination; Heather Raley, MA, for participant recruitment; and Hailey Turner, BS, and Jared Linthicum, MS, for help with the data collection (all are affiliated with Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine, Baltimore). None of the acknowledged individuals report any conflicts of interest related to the subject of this letter.
Previous presentation: Dr Koola presented the data at the 51st American College of Neuropsychopharmacology meeting; December 2-6, 2012; Hollywood, Florida.
Published online: September 1, 2016.
Prim Care Companion CNS Disord 2016;18(5):doi:10.4088/PCC.16l01959
© Copyright 2016 Physicians Postgraduate Press, Inc.
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top