ABSTRACT
Objective: To better understand Takotsubo cardiomyopathy, a rare but life-threatening complication of acute substance withdrawal.
Data Sources: A PubMed search was conducted to identify relevant case reports through 2021 using the medical subject headings alcohol withdrawal, opioid withdrawal, benzodiazepine withdrawal OR withdrawal AND Takotsubo OR stress cardiomyopathy.
Study Selection: Case reports were included in the review if there was a diagnosis of Takotsubo cardiomyopathy in the setting of withdrawal from substances of abuse. Case reports were excluded if patients were withdrawing from other substances, actively intoxicated, or had myocardial ischemia.
Data Extraction and Synthesis: Data were manually abstracted for the variables of interest, including demographics, symptoms, medical evaluation, and treatment. Descriptive statistics of the demographics, symptoms, medical evaluation, and treatment of Takotsubo cardiomyopathy were analyzed.
Results: The mean (SD) age of patients experiencing withdrawal-associated Takotsubo cardiomyopathy was 50.8 years (15.2), and 64% of patients were female. The most common signs and symptoms were tachycardia (60%), changes in blood pressure (48%), altered mental status (48%), dyspnea (32%), nausea or vomiting (28%), and chest pain (28%). All patients with a reported electrocardiogram (92%) demonstrated ECG abnormalities; 76% had an elevated troponin level, and 24% had an elevated CK-MB level. Medications that could treat withdrawal and α2-agonists were utilized for 60% and 12% of patients, respectively. Ventilator support, cardiopulmonary resuscitation, and intra-aortic balloon pump were needed for 24%, 8%, and 8%, respectively, of patients with withdrawal-associated Takotsubo cardiomyopathy.
Conclusions: Withdrawal-associated Takotsubo cardiomyopathy is a rare but potentially life-threatening complication of substance withdrawal. Clinicians should maintain a high degree of clinical suspicion for withdrawal-associated Takotsubo in patients with a history of substance use disorders or physical dependence on benzodiazepines or opioids, as the clinical presentation may be atypical.
Prim Care Companion CNS Disord 2023;25(1):22r03304
To cite: Back DK, Bui AH, Ahmed S, et al. Substance withdrawal–associated Takotsubo cardiomyopathy: a review of the literature. Prim Care Companion CNS Disord. 2023;25(1):22r03304.
To share: https://doi.org/10.4088/PCC.22r03304
© 2023 Physicians Postgraduate Press, Inc.
aDepartment of Medicine, Massachusetts General Hospital, Boston, Massachusetts
bVirginia Cardiovascular Specialists, Richmond, Virginia
cWest Ridge Center for Addiction Recovery, Rutland Regional Medical Center, Rutland, Vermont
dDepartment of Psychiatry, Brigham and Women’s Hospital, Boston, Massachusetts
*Corresponding author: Saeed Ahmed, MD, West Ridge Center for Addiction Recovery, Rutland Regional Medical Center, 160 Allen St, Rutland, VT 05701 ([email protected]).
An estimated 21.5 million Americans—8.1% of the US population—have substance use disorders.1 According to the most recent data from the National Center for Health Statistics, there were 100,306 drug overdose deaths in the United States during the 12-month period ending April 2021, a 28.5% increase from the 78,056 deaths the previous year.2 In the inpatient setting, approximately 20% and 4% of hospitalized patients have an alcohol use disorder or opioid use disorder, respectively.3,4 Withdrawal from alcohol or benzodiazepines can have deadly consequences including seizures and delirium tremens,5 but withdrawal from opioids is generally not thought to be life threatening. In the past few years, however, there have been numerous case reports of Takotsubo cardiomyopathy developing in the setting of acute withdrawal from alcohol, benzodiazepines, or opioids. This cardiac involvement marks a potentially new symptom of substance withdrawal: withdrawal-associated Takotsubo cardiomyopathy.
Despite individual case reports of Takotsubo cardiomyopathy occurring in the setting of withdrawal from alcohol, benzodiazepines, or opioids, there has not yet been a review of all cases of withdrawal-associated Takotsubo cardiomyopathy. We aimed to analyze the demographics, symptoms, medical evaluation, and treatment approaches for this rare complication of withdrawal. We believe that Takotsubo cardiomyopathy should be included in the differential for patients who develop atypical signs or symptoms while withdrawing from alcohol, benzodiazepines, or opioids. Takotsubo cardiomyopathy is a treatable condition that should improve with medical treatment of withdrawal.
METHODS
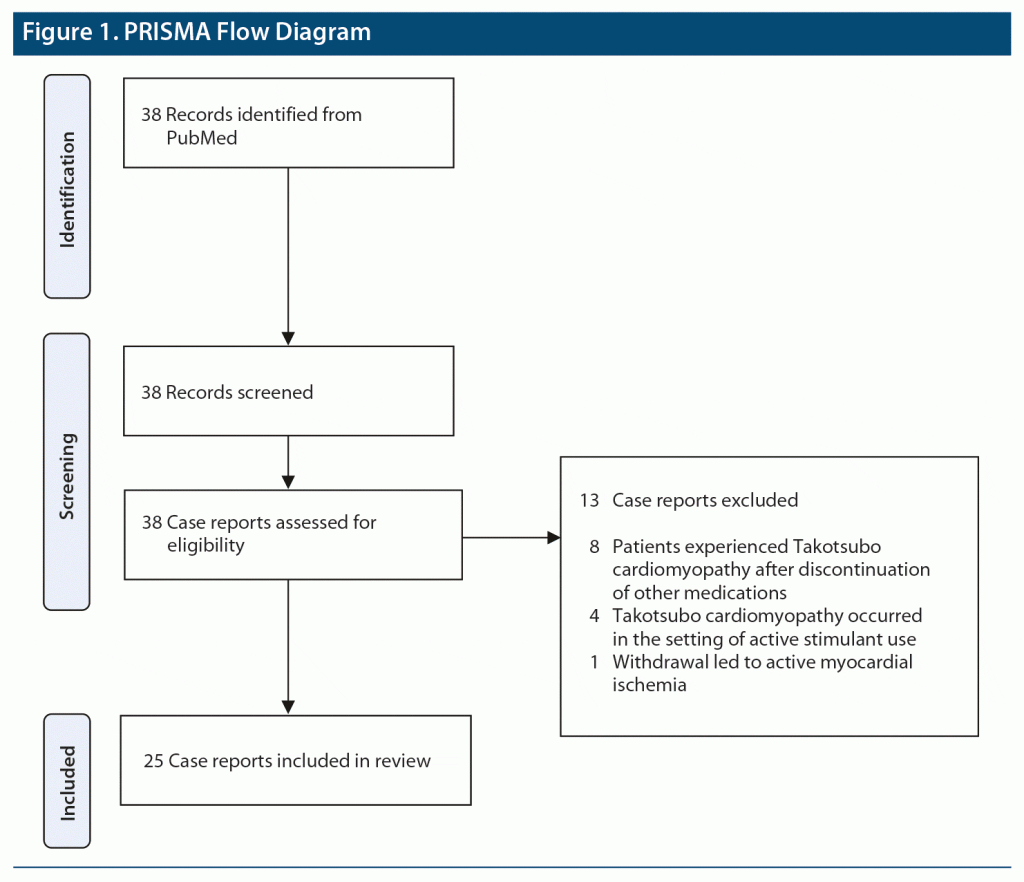
A PubMed search was conducted to identify relevant case reports through 2021 using the medical subject headings alcohol withdrawal, opioid withdrawal, benzodiazepine withdrawal OR withdrawal AND Takotsubo OR stress cardiomyopathy. Case reports were included in the review if there was a diagnosis of Takotsubo cardiomyopathy in the setting of withdrawal from substances of abuse. Case reports were excluded if patients were withdrawing from other substances or actively intoxicated. Thirty-eight case reports were identified with these search criteria, but 13 cases did not meet the inclusion criteria and were excluded (Figure 1). Eight cases were excluded because patients experienced Takotsubo cardiomyopathy after discontinuation of other medications, such as metoprolol, baclofen, and antidepressants. Four other cases were excluded due to Takotsubo occurring in the setting of active stimulant use. One case was excluded as the withdrawal led to active myocardial ischemia. The remaining 25 cases were included in the review.6–30
Data on the most common physical examination findings, laboratories, imaging, and treatment approaches were abstracted. Data were also abstracted for any prior cardiac history, defined as prior history of myocardial infarction, structural heart defects, heart failure, or arrhythmia. Descriptive statistics were then analyzed using STATA 14.1. Continuous variables are presented as means with standard deviations, and categorical variables are presented as percentages.
RESULTS
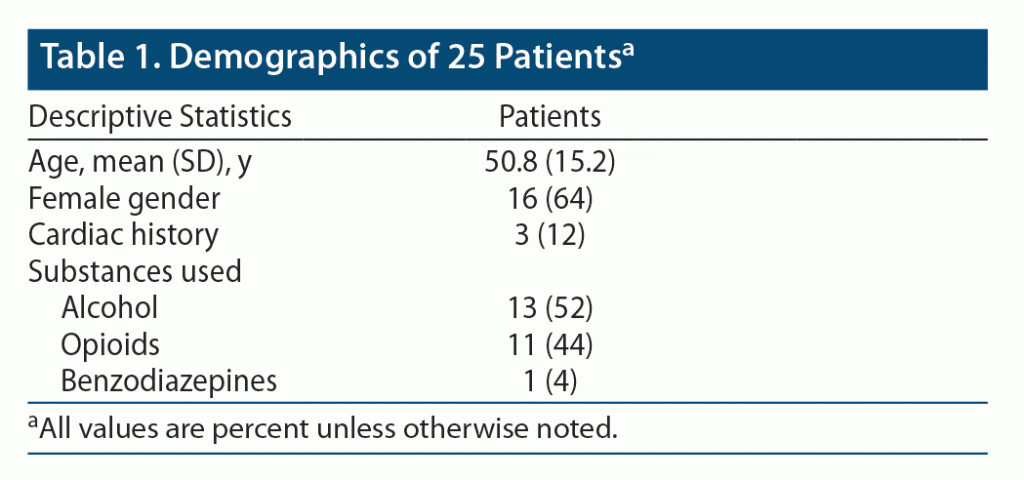
The characteristics of patients with withdrawal-associated Takotsubo cardiomyopathy are reported in Table 1. The mean (SD) age was 50.8 (15.2) years, and 64% of patients were female. Most patients withdrew from alcohol (52%) or opioids (44%), but a smaller number withdrew from benzodiazepines (4%). Only 3 patients reported a prior cardiac history.
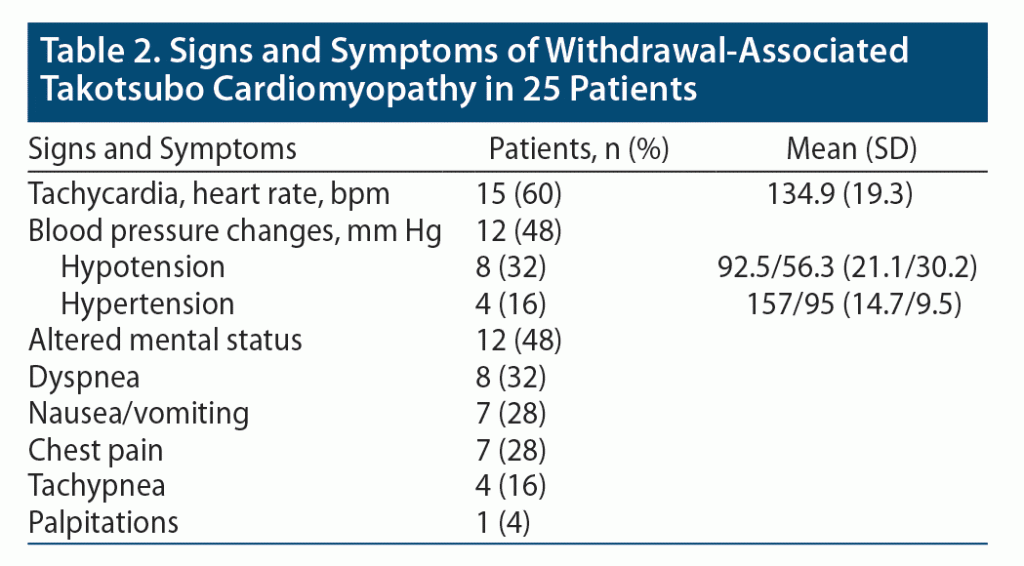
The most common signs and symptoms in withdrawal-associated Takotsubo cardiomyopathy are illustrated in Table 2. Fifteen patients (60%) were tachycardic, with a mean (SD) heart rate of 135 (19.3) bpm. Twelve patients (48%) experienced changes in blood pressure; 8 (32%) were hypotensive, while 4 (16%) were hypertensive. Twelve individuals (48%) were found to have altered mental status. Only 8 (32%) endorsed dyspnea, 7 (28%) reported nausea or vomiting, 7 (28%) experienced chest pain, and 1 (4%) felt palpitations. Four patients (16%) were hypoxic, with a mean oxygen saturation of 85.4%. Four patients (16%) were tachypneic. Withdrawal symptoms were not consistently documented in the case reports.
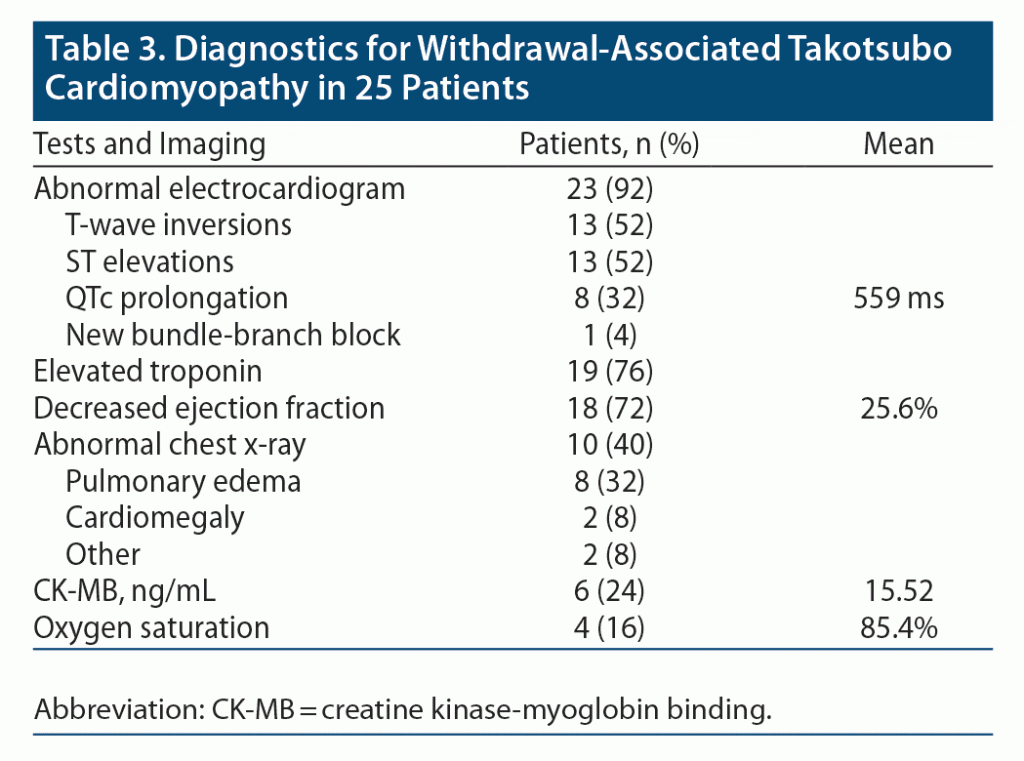
Laboratory tests and imaging results are displayed in Table 3. All patients with withdrawal-associated Takotsubo and a reported electrocardiogram (ECG; 92%) had ECG abnormalities; 13 patients (52%) had T-wave inversions, 13 (52%) had ST elevations, 8 (32%) had QTc prolongation, and 1 (4%) had a new bundle-branch block. When QTc interval prolongation was present, the mean prolonged QTc interval was 559 ms. Additionally, 19 patients (76%) had an elevated troponin level, while 6 patients (24%) had an elevated CK-MB (creatine kinase-myoglobin binding ratio) level. Eighteen patients (72%) underwent echocardiography. Of those with available reported echocardiography, the mean left ventricular ejection fraction was 25.6%. Chest x-rays were abnormal for 10 patients (40%). The most common abnormal finding was pulmonary edema (32%), followed by cardiomegaly (8%), lung consolidation (4%), and other findings (8%).
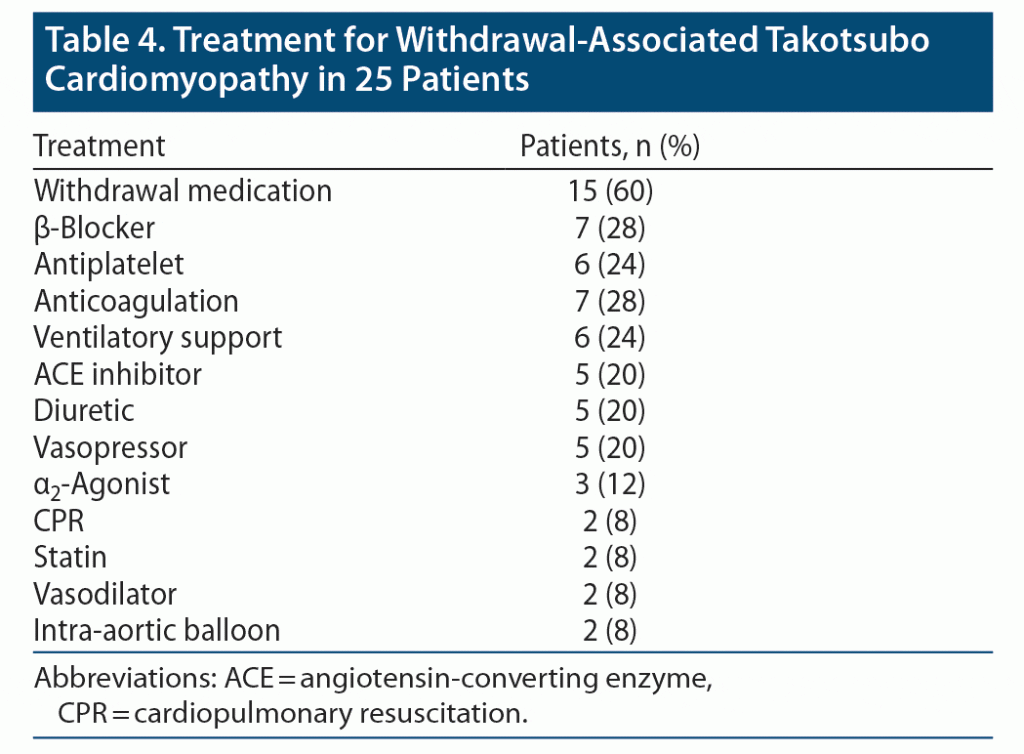
Finally, medications used during management of withdrawal-associated Takotsubo cardiomyopathy are shown in Table 4. The most used cardiac medications were β-blockers (28%), anticoagulation (28%), antiplatelet therapy (24%), ACE inhibitors (20%), diuretics (20%), vasopressors (20%), statins (8%), and vasodilators (8%). Medications that could treat withdrawal, whether purposively or as part of a pain management strategy, were utilized in 60% of cases. α2-Agonists were implemented 12% of the time. Rarer interventions included ventilator support (24%), cardiopulmonary resuscitation (8%), and intra-aortic balloon pump (8%).
DISCUSSION
Takotsubo cardiomyopathy, or stress cardiomyopathy, was first described in the Japanese population by Sato in 1990.31 It was named after the octopus trapping pot with a round bottom and narrow neck, resembling the left ventricular apical ballooning that can be seen during the left ventriculogram. Takotsubo cardiomyopathy is a distinct reversible cardiomyopathy that is classically triggered by emotional stress.32 However, studies have attributed the development of Takotsubo cardiomyopathy following physical stressors (including the postoperative period33), a variety of medical conditions (including subarachnoid hemorrhage,34,35 epilepsy,36 trauma,37 and thyrotoxicosis38), and pharmacologic agents (including general anesthesia,39 catecholamines,40 and chemotherapy41). It appears that Takotsubo cardiomyopathy can also be triggered by acute withdrawal from substances of misuse including but not limited to alcohol, benzodiazepines, and opioids.
While the mechanism for Takotsubo cardiomyopathy is not completely understood, a catecholamine excess is implicated in stunning the myocardium, either through multivessel epicardial coronary artery spasm, microvascular spasm, or direct myocyte injury.42,43 Withdrawal from alcohol, benzodiazepines, and opioids has been shown to be a highly adrenergic state with increased levels of catecholamines.44–46 Thus, it is possible that this catecholamine excess in the setting of withdrawal results in cardiac stunning, which then creates transient systolic and diastolic left ventricular dysfunction with wall motion abnormalities.47 Patients with Takotsubo cardiomyopathy present with signs of left ventricular dysfunction, but have normal coronary arteriography results and usually recover cardiac function within days or weeks.47 While this cardiomyopathy was previously thought to have a good prognosis due to its reversible nature, subsequent studies have shown that it might not have a benign course. Approximately 4.5% of patients with Takotsubo will die during their hospitalization, and 4% will die within 30 days of discharge.48,49 In a cohort of 1,750 patients, Templin et al50 described a rate of 21.8% with a combined end point of serious in-hospital complications, long-term follow-up with rate of death per patient-year of 5.6%, and a rate of stroke or transient ischemic attack of 1.7% per patient-year.
In this review, demographics for patients with non–withdrawal-associated and withdrawal-associated Takotsubo cardiomyopathy differed moderately. Patients with non–withdrawal-associated Takotsubo cardiomyopathy are overwhelmingly female and elderly. In a study of over 1,000 patients with Takotsubo cardiomyopathy, 89.9% were female, and there was a mean age of 66.8 years.50 However, in our review of withdrawal-associated Takotsubo cases, a smaller proportion of patients were female (65.2%), and the mean age was 50.7 years.
In addition to the potential differences in demographics, the clinical presentation for those with withdrawal-associated Takotsubo may differ from non–withdrawal-associated Takotsubo. While patients with non–withdrawal-associated Takotsubo cardiomyopathy most commonly present with chest pain and dyspnea,47,50 patients with withdrawal-associated Takotsubo cardiomyopathy presented primarily with withdrawal symptoms such as tachycardia (65.2%), changes in blood pressure (47.8%), and altered mental status (47.8%). Chest pain (26.1%), nausea (26.1%), and dyspnea (34.8%) were less commonly reported symptoms for patients with withdrawal-associated Takotsubo cardiomyopathy, perhaps due to an inability to express these subjective symptoms in the setting of acute withdrawal. Accordingly, the absence of a complaint of chest pain or an anginal equivalent like nausea or dyspnea in a patient undergoing acute withdrawal does not exclude Takotsubo cardiomyopathy.
While demographics and clinical presentations may differ, laboratory testing and imaging for withdrawal-associated Takotsubo were largely similar to that of non–withdrawal associated Takotsubo. Because withdrawal-associated Takotsubo cardiomyopathy is underrecognized, clinicians should maintain a high degree of clinical suspicion in patients with a history of substance use disorders or physical dependence on benzodiazepines or opioids. Patients in withdrawal may be unable to report physical symptoms like cardiac pain or dyspnea, but clinicians should nonetheless include Takotsubo cardiomyopathy in their differential diagnosis for patients with substance use disorders or physical dependence on opioids or benzodiazepines who present with altered mental status and vital sign abnormalities.
There is no universally recommended treatment for Takotsubo cardiomyopathy. β-Blockers have been proposed as one treatment approach,51 since they counter the catecholamine excess that has been implicated in the pathophysiology of Takotsubo cardiomyopathy. Other treatment approaches during hospitalization include measures to support acute decompensated heart failure with intravenous (IV) diuretics, IV inotropes, and IV nitrates.49 Despite the development of Takotsubo cardiomyopathy in the setting of acute withdrawal, treatment for withdrawal was reported for only 60% of cases. Because the withdrawal treatment and severity were not always reported, it was not possible to evaluate the efficacy of these patients’ withdrawal treatment. However, we believe that adequate withdrawal management is an integral component of care for withdrawal-associated Takotsubo, since untreated or undertreated withdrawal could prolong the state of catecholamine excess resulting in further catecholamine-mediated cardiotoxicity. In addition to providing withdrawal treatment for patients with withdrawal-associated Takotsubo cardiomyopathy, clinicians should view these hospitalizations as opportunities to initiate substance use disorder treatment.49
This study has several limitations. It is limited by a relatively small sample size, the variability in reported variables in the published case reports, and the lack of long-term patient follow-up. It is further limited by the inconsistent reporting of withdrawal severity and treatment. Additionally, we cannot definitively conclude that withdrawal causes Takotsubo cardiomyopathy; instead, we note that there is an association between withdrawal and stress cardiomyopathy. However, this study also has many strengths. We included all available case reports of withdrawal-associated Takotsubo cardiomyopathy in the medical literature and analyzed all the available data in these case reports. We also provided insight into the potential pathophysiology of withdrawal-associated Takotsubo cardiomyopathy and suggest a clinical approach for treating patients with this complication.
This review demonstrates that patients with substance use disorders and those prescribed opioids and benzodiazepines are at risk for withdrawal-associated Takotsubo cardiomyopathy. Adequate withdrawal management should be included in any treatment of withdrawal-associated Takotsubo cardiomyopathy, since untreated or undertreated withdrawal could lead to a surge of catecholamines responsible for the cardiomyopathy. Future studies are needed to determine the incidence, pathophysiology, and therapeutic approach to the management of withdrawal-associated Takotsubo cardiomyopathy.
Submitted: April 12, 2022; accepted July 21, 2022.
Published online: February 21, 2023.
Relevant financial relationships: None.
Funding/support: None.
Clinical Points
- Withdrawal-associated Takotsubo cardiomyopathy should be suspected when patients with a history of substance use disorders or physical dependence on benzodiazepines or opioids develop atypical symptoms in the setting of withdrawal, including tachycardia, changes in blood pressure, chest pain, dyspnea, and altered mental status.
- Appropriate withdrawal should be considered in the treatment of withdrawal-associated Takotsubo cardiomyopathy, since untreated or inadequately treated withdrawal may result in a surge of catecholamines responsible for cardiotoxicity.
References (51)

- Hedden SL. Behavioral Health Trends in the United States: Results From the 2014 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration, Department of Health and Human Services. Accessed January 10, 2023. https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf
- Centers for Disease Control and Prevention. Drug overdose deaths in the US Top 100,000 annually. Accessed January 10, 2023. https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm
- O’Connor PG, Schottenfeld RS. Patients with alcohol problems. N Engl J Med. 1998;338(9):592–602. PubMed CrossRef
- McNeely J, Gourevitch MN, Paone D, et al. Estimating the prevalence of illicit opioid use in New York City using multiple data sources. BMC Public Health. 2012;12(1):443. PubMed CrossRef
- Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348(18):1786–1795. PubMed CrossRef
- Suzuki K, Osada N, Akasi YJ, et al. An atypical case of “Takotsubo cardiomyopathy” during alcohol withdrawal: abnormality in the transient left ventricular wall motion and a remarkable elevation in the ST segment. Intern Med. 2004;43(4):300–305. PubMed CrossRef
- Maruyama S, Nomura Y, Fukushige T, et al. Suspected takotsubo cardiomyopathy caused by withdrawal of bupirenorphine in a child. Circ J. 2006;70(4):509–511. PubMed CrossRef
- Mitchell SA, Crone RA. Takotsubo cardiomyopathy: a case report. J Am Soc Echocardiogr. 2006;19(9):1190.e9–1190.e10. PubMed CrossRef
- Rivera JM, Locketz AJ, Fritz KD, et al. “Broken heart syndrome” after separation (from OxyContin). Mayo Clin Proc. 2006;81(6):825–828. PubMed CrossRef
- Kalra N, Khetpal P, Sorrell VL. Seriously stressed. Am J Med. 2009;122(8):735–737. PubMed CrossRef
- Yousuf MA, Adjei S, Kinder B. A 58-year-old woman with ST-segment elevation, seizures, and altered mental status in the setting of opiate withdrawal. Chest. 2009;135(4):1098–1101. PubMed CrossRef
- Lemesle F, Lemesle F, Nicola W, et al. First case of stress cardiomyopathy as a result of methadone withdrawal secondary to drug-drug interaction. Am J Emerg Med. 2010;28(3):387.e5–387.e6. PubMed CrossRef
- Alexandre J, Benouda L, Champ-Rigot L, et al. Takotsubo cardiomyopathy triggered by alcohol withdrawal. Drug Alcohol Rev. 2011;30(4):434–437. PubMed CrossRef
- Stöllberger C, Wegner C, Finsterer J. Seizure-induced Takotsubo syndrome is more frequent than reported. Int J Cardiol. 2011;150(3):359–360. PubMed CrossRef
- Thompson AG, Hung J. Takotsubo cardiomyopathy associated with alcohol withdrawal. Med J Aust. 2011;194(7):373. PubMed CrossRef
- Stout BJ, Hoshide R, Vincent DS. Takotsubo cardiomyopathy in the setting of acute alcohol withdrawal. Hawaii J Med Public Health. 2012;71(7):193–194. PubMed
- Chan JCM, Wong RSM, Chan TYK. Takotsubo cardiomyopathy associated with sudden benzodiazepine withdrawal. Clin Toxicol. 2012;50(4):323.
- Omar HR, Abdelmalak HD, Komorova I, et al. Alcohol withdrawal-induced Takotsubo. Intern Emerg Med. 2012;7(suppl 2):S107–S108. PubMed CrossRef
- Yazdan-Ashoori P, Nichols R, Baranchuk A. Takotsubo cardiomyopathy precipitated by alcohol withdrawal. Cardiol J. 2012;19(1):81–85. PubMed CrossRef
- Saiful FB, Lafferty J, Jun CH, et al. Takotsubo cardiomyopathy due to iatrogenic methadone withdrawal. Rev Cardiovasc Med. 2011;12(3):164–167. PubMed CrossRef
- Moore PK, Marzec LN, Krantz MJ. Signs of stress: deep symmetric T-wave inversions. Am J Med. 2013;126(8):682–684. PubMed CrossRef
- Revelo AE, Pallavi R, Espana-Schmidt C, et al. ‘Stoned’ people can get stunned myocardium: a case of heroin withdrawal precipitating Tako-Tsubo cardiomyopathy. Int J Cardiol. 2013;168(3):e96–e98. PubMed CrossRef
- Spadotto V, Zorzi A, Elmaghawry M, et al. Heart failure due to ‘stress cardiomyopathy’: a severe manifestation of the opioid withdrawal syndrome. Eur Heart J Acute Cardiovasc Care. 2013;2(1):84–87. PubMed CrossRef
- Sarcon A, Ghadri JR, Wong G, et al. Takotsubo cardiomyopathy associated with opiate withdrawal. QJM. 2014;107(4):301–302. PubMed CrossRef
- Agu CC, Bakhit A, Basunia M, et al. Takotsubo cardiomyopathy precipitated by delirium tremens. J Community Hosp Intern Med Perspect. 2015;5(6):29704. PubMed CrossRef
- Harris ZM, Alonso A, Kennedy TP. Adrenergic inhibition with dexmedetomidine to treat stress cardiomyopathy during alcohol withdrawal: a case report and literature review. Case Rep Crit Care. 2016;2016:9693653. PubMed CrossRef
- Olson PC, Agarwal V, Lafferty JC, et al. Takotsubo cardiomyopathy precipitated by opiate withdrawal. Heart Lung. 2018;47(1):73–75. PubMed CrossRef
- Surmaitis RM, Khalid MM, Vearrier D, et al. Takotsubo cardiomyopathy associated with buprenorphine precipitated withdrawal. Clin Toxicol (Phila). 2018;56(9):863–864. PubMed CrossRef
- Kulkarni M, Kulkarni A, Healy J, et al. Acute alcohol withdrawal-induced Takotsubo cardiomyopathy: a rare presentation. Chest. 2019;156(4):A2190–A2191. CrossRef
- Makani A, Genuardi M, Sciortino C, et al. Opioid withdrawal induced stress cardiomyopathy with recurrent cardiogenic shock. J Am Coll Cardiol. 2019;73(9 suppl 1):2207. CrossRef
- Sato H. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm. Clin Asp Myocard Inj from ischemia to Hear Fail. 1990:56–64.
- Brandspiegel HZ, Marinchak RA, Rials SJ, et al. A broken heart. Circulation. 1998;98(13):1349. PubMed CrossRef
- Deniz S, Bakal Ö, İnangil G, et al. Takotsubo cardiomyopathy occurring in the postoperative period. Turk J Anaesthesiol Reanim. 2015;43(1):47–49. PubMed CrossRef
- Mayer SA, Lin J, Homma S, et al. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30(4):780–786. PubMed CrossRef
- Lee VH, Connolly HM, Fulgham JR, et al. Tako-tsubo cardiomyopathy in aneurysmal subarachnoid hemorrhage: an underappreciated ventricular dysfunction. J Neurosurg. 2006;105(2):264–270. PubMed CrossRef
- Mugnai G, Pasqualin G, Prati D, et al. Recurrent multiform Takotsubo cardiomyopathy in a patient with epilepsy: broken heart or brain? Int J Cardiol. 2015;201:332–335. PubMed CrossRef
- Vergez M, Pirracchio R, Mateo J, et al. Tako Tsubo cardiomyopathy in a patient with multiple trauma. Resuscitation. 2009;80(9):1074–1077. PubMed CrossRef
- Eliades M, El-Maouche D, Choudhary C, et al. Takotsubo cardiomyopathy associated with thyrotoxicosis: a case report and review of the literature. Thyroid. 2014;24(2):383–389. PubMed CrossRef
- Seno H, Komasawa N, Hasegawa Y, et al. Development of Takotsubo cardiomyopathy during recovery from general anesthesia: a case report. Masui. 2015;64(8):826–829. PubMed
- Law C, Khaliq A, Guglin M. Reversible cardiogenic shock due to catecholamine-induced cardiomyopathy: a variant of takotsubo? Am J Emerg Med. 2013;31(11):1621.e1–1621.e3. PubMed CrossRef
- Goel S, Sharma A, Garg A, et al. Chemotherapy induced Takotsubo cardiomyopathy. World J Clin Cases. 2014;2(10):565–568. PubMed CrossRef
- Wittstein IS, Thiemann DR, Lima JAC, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539–548. PubMed CrossRef
- Naegele M, Flammer AJ, Enseleit F, et al. Endothelial function and sympathetic nervous system activity in patients with Takotsubo syndrome. Int J Cardiol. 2016;224:226–230. PubMed CrossRef
- Borg S, Czarnecka A, Kvande H, et al. Clinical conditions and concentrations of MOPEG in the cerebrospinal fluid and urine of male alcoholic patients during withdrawal. Alcohol Clin Exp Res. 1983;7(4):411–415. PubMed CrossRef
- Bell J, Bickford-Wimer PC, de la Garza R, et al. Increased central noradrenergic activity during benzodiazepine withdrawal: an electrophysiological study. Neuropharmacology. 1988;27(11):1187–1190. PubMed CrossRef
- McDonald T, Hoffman WE, Berkowitz R, et al. Heart rate variability and plasma catecholamines in patients during opioid detoxification. J Neurosurg Anesthesiol. 1999;11(3):195–199. PubMed CrossRef
- Pelliccia F, Kaski JC, Crea F, et al. Pathophysiology of Takotsubo syndrome. Circulation. 2017;135(24):2426–2441. PubMed CrossRef
- Singh K, Carson K, Shah R, et al. Meta-analysis of clinical correlates of acute mortality in takotsubo cardiomyopathy. Am J Cardiol. 2014;113(8):1420–1428. PubMed CrossRef
- Redfors B, Vedad R, Angerås O, et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction: a report from the SWEDEHEART registry. Int J Cardiol. 2015;185:282–289. PubMed CrossRef
- Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929–938. PubMed CrossRef
- Kyuma M, Tsuchihashi K, Shinshi Y, et al. Effect of intravenous propranolol on left ventricular apical ballooning without coronary artery stenosis (ampulla cardiomyopathy): three cases. Circ J. 2002;66(12):1181–1184. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top