ABSTRACT
Objective: To determine if superior temporal gyrus volumes are altered in patients with a social anxiety disorder.
Methods: Magnetic resonance imaging (MRI) was utilized to determine the superior temporal gyrus volume in 21 patients with a social anxiety disorder and 20 control subjects without a social anxiety disorder. The superior temporal gyrus volumes were measured by manual tracing method. The study was conducted between September 2019 and April 2020.
Results: The mean superior temporal gyrus volume for both sides was statistically significantly smaller than that of control subjects (left side: 11.38 ± 0.85 cm3 for patients and 12.73 ± 0.86 cm3 for controls, t=–5.064, P < .001; right side: 11.42 ± 0.84 mm3 for patients and 12.92 ± 0.85 cm3 for controls, t=–5.574, P < .001). Moreover, when comparing volumetric measurements for subregions, we detected that volumes of all subregions were also statistically significantly smaller than those of healthy comparisons (for both sides of the Heschl’s gyrus and planum temporale).
Conclusions: The study findings suggest that patients with social anxiety disorder seem to have smaller superior temporal gyrus volumes compared to healthy control subjects, although we do not know whether these results were in accordance with functional changes of the same region.
Prim Care Companion CNS Disord 2021;23(5):20m02815
To cite: Atmaca M, Koc M, Aslan S, et al. Superior temporal gyrus volumes in patients with social anxiety disorder. Prim Care Companion CNS Disord. 2021;23(5):20m02815.
To share: https://doi.org/10.4088/PCC.20m02815
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, School of Medicine, Firat University, Elazig, Turkey
bDepartment of Radiology, School of Medicine, Firat University, Elazig, Turkey
*Corresponding author: Murad Atmaca, MD, Firat (Euphrates) Universitesi, Firat Tip Merkezi, Psikiyatri Anabilim Dali 23119, Elazig, Turkey ([email protected]).
Social anxiety disorder, also called social phobia, is a psychiatric disorder included with anxiety disorders in the DSM-5.1 It is described as an intense, persistent fear of being watched and judged by others. Patients with social anxiety disorder have fear of 1 or more social or performance situations in which the person is exposed to unfamiliar people or possible scrutiny by others. This persistent fear can influence the patient’s work, school life, and daily activities. Patients with social anxiety disorder demonstrate physiologic symptoms such as sweating, palpitations, nausea, trembling, shaking, and blushing when exposed to the feared social conditions. It is well established that social anxiety disorder is one of the most frequent psychiatric disorders.2 In a national field study,3 the prevalence of social anxiety disorder was reported to be 13.3%. The exact etiopathogenesis of social anxiety disorder has not been established. However, genetic neurobiological studies and neuroimaging investigations revealed important findings. Previously, we4 investigated patients with social anxiety disorder, examining the activity levels of antioxidant enzymes (superoxide dismutase, SOD; glutathione peroxidase, GSH-Px; and catalase, CAT) and malondialdehyde (MDA), a product of lipid peroxidation. In that study,4 our team determined that the mean MDA, SOD, GSH-Px, and CAT values in patients with social anxiety disorder were statistically significantly increased compared to those of healthy controls. A positive correlation was found between Liebowitz Social Anxiety Scale scores and MDA, SOD, and GSH-Px levels and between duration of illness and MDA, SOD, and CAT levels in patients, and it was noted that free radical damage might be associated with the pathophysiology of social anxiety disorder.4
When searching the literature, a limited number of neuroimaging studies exist on patients with social anxiety disorder, although remarkable results have been reported, which provide a better understanding of the neuroanatomic nature of the disorder. In a pioneering neuroimaging investigation, Potts et al5 found that no statistically significant volumetric differences existed between patients with social anxiety disorder and healthy control subjects. In another study,6 researchers measured the hippocampus and amygdala volumes of patients with social anxiety disorder and reported that volumes of the regions of interest were significantly smaller in patients with social anxiety disorder compared to those of healthy control subjects. They6 also found negative correlations between right hippocampus volumes and Liebowitz Social Anxiety Scale scores and between right amygdala volumes and State-Trait Anxiety Inventory scores. On the other hand, significant subcortical volumetric changes in a variety of structures consisting of both sides of the thalamus, right amygdala, and right precuneus, with a negative relationship between the right amygdala volumes and duration of illness, were reported by another study.5 Our study team7 also carried out a magnetic resonance imaging (MRI) investigation on the hippocampus and amygdala volumes of 22 patients with social anxiety disorder and the same number of healthy controls and found that hippocampus volumes in both sides of the patients were statistically significantly greater than those of the healthy controls, while there were no volumetric differences in amygdala volumes. In an unpublished study, we measured pituitary volumes in patients with social anxiety disorder and healthy controls and determined that the patients had a statistically significantly smaller mean pituitary gland volume compared to controls, which was also supported by an analysis of covariance (ANCOVA) undercontrolling for age, sex, and total brain volumes [Murad Atamaca, MD, unpublished data, 2021]. In that study, we concluded that patients with a social anxiety disorder could be associated with smaller pituitary gland volumes and that these reduced volumes might be related to anxiety itself, considering that our previous pituitary volume studies in a variety of anxiety and anxiety-related disorders found similar results. Finally, in another study we examined insula volumes in patients with social anxiety disorder compared with healthy control subjects [Murad Atamaca, MD, unpublished data, 2021]. In that study, we detected that the mean right and left posterior and anterior insula volumes of patients were statistically significantly reduced compared to those of healthy control subjects.
The superior temporal gyrus is 1 of 3 gyri in the temporal lobe of the brain that is placed laterally to the head. It consists of some important brain regions including Brodmann’s 41 and 42, responsible for the sensation of sounds, and Wernicke’s area, responsible for the understanding of speech.8 The superior temporal gyrus is associated with auditory processing, including language, but also has been emphasized as a critical structure in social cognition.8 The superior temporal gyrus has been implicated as an important structure in the pathway containing the amygdala and prefrontal cortex, which are all involved in social cognition processes.9 Moreover, it has been reported that the superior temporal gyrus is involved in the perception of emotions in facial stimuli, which is related to the clinical presentation of patients with a social anxiety disorder.10 Thus, the superior temporal gyrus seems to be able to associate with the neuroanatomy of social anxiety disorder. However, there has not been a study, to our knowledge, on volumes of the superior temporal gyrus in patients with social anxiety disorder. For this reason, we aimed to examine superior temporal gyrus volumes by using the manual tracing method in patients with a social anxiety disorder and hypothesized that superior temporal gyrus volumes would be altered in this group.
METHODS
Subjects
The study included 21 patients with a social anxiety disorder from the inpatient or outpatient clinics of the Department of Psychiatry, Firat University School of Medicine, Elazig, Turkey. The patients were aged 18–65 years (mean ± SD age = 29.46 ± 6.12 years) and met DSM-IV-TR criteria for a social anxiety disorder with the Structured Clinical Interview for DSM-IV-TR (SCID).11
Exclusion criteria for patients included the presence of any congenital malformation and congenital brain malformations that can influence the measurements of regions such as arachnoid cyst, any current or lifetime neurologic or endocrinologic conditions, the existence of current severe medical problems and mental retardation, any medical problems (eg, cardiac, joint) that would interfere with the neuroimaging examination, a low level of education limiting a satisfying interview, and alcohol/substance abuse within the 6 months preceding the study. We did not exclude depression, because depression is the comorbid condition most frequently seen in patients with a social anxiety disorder.
Twenty healthy control subjects with a mean ± SD age of 30.89 ± 4.26 years were also included in the study. Exclusion criteria for healthy control subjects included any DSM-IV Axis I disorder in self or a first-degree relative as determined by the SCID nonpatient version, presence of any current medical problems (including endocrinologic or neurologic) or psychiatric histories, any use of psychoactive medication within 2 weeks, any congenital malformation on brain imaging, any problems limiting the neuroimaging examination such as cardiac or joint condition, a low level of education limiting the interview, and alcohol/substance abuse within the 6 months preceding the study.
All study procedures were carried out in accordance with the guidelines of the Declaration of Helsinki. The study was approved by the Firat University School of Medicine Local Ethics Committee, and written informed consent was obtained from all study subjects. The study was conducted between September 2019 and April 2020.
MRI Procedure
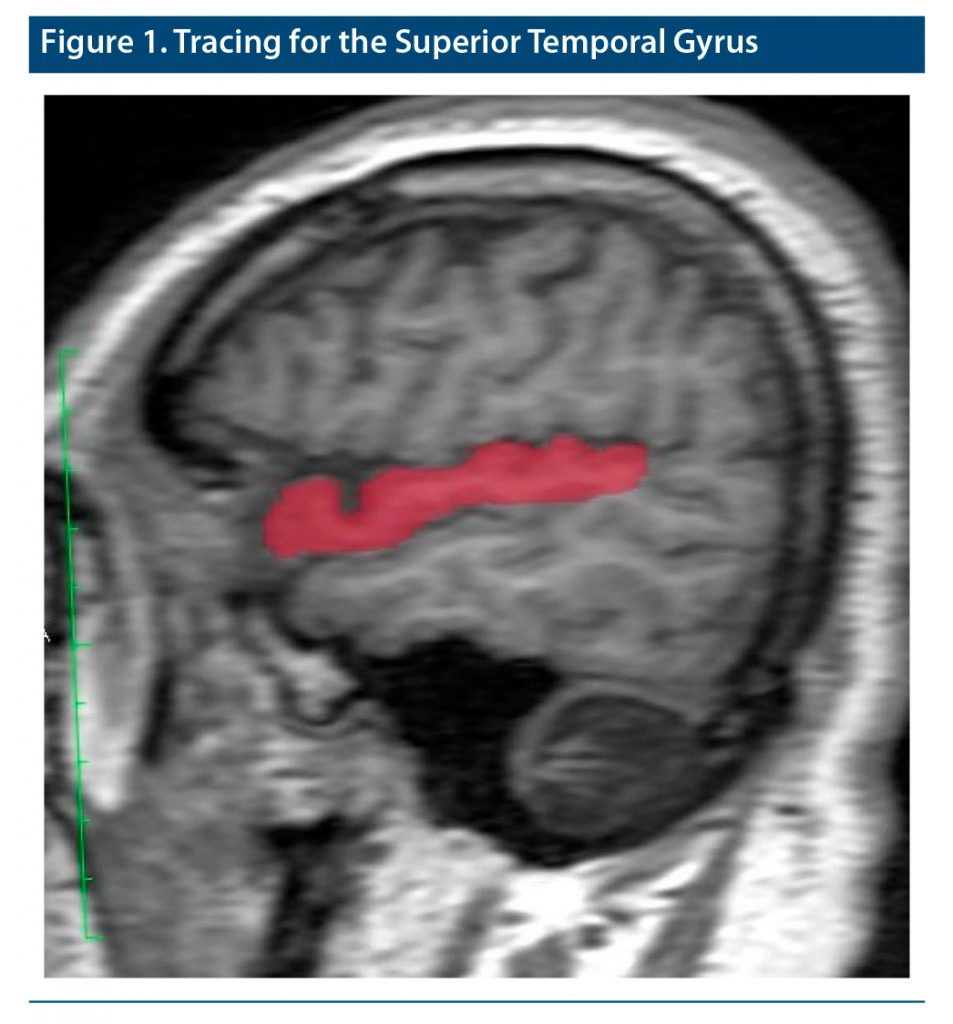
The 1.5-T GE scanner (GE Healthcare, Milwaukee, Wisconsin) was used to obtain the brain MRIs of patients and controls. We used an 8-channel head coil to obtain brain imaging. All subjects were told they could take an anxiolytic agent to reduce their worry and avoidance during the imaging process. High-resolution structural T1-weighted images were obtained from all subjects using sagittally acquired 3D spiral fast spin-echo high-resolution images. During the imaging process, some MRI parameters were selected, including repetition time of 14.4 ms, number of excitations of 1, echo time of 15.6 ms, band diameter of 20.8, field of view of 240 mm, rotation angle of 20°, and resolution of 0.9375 × 0.9375 × 2.4 mm. Meanwhile, after the tracing process all measurements were carried out by utilizing a computer-advanced workstation with the GE Healthcare Volume Viewer voxtool 4.6 program. As in our previous MRI investigations, all tracings of the superior temporal gyrus regions were carried out by a neuroradiologist. The neuroradiologist was blind to diagnosis and did not know whether the subject was from the patient or control group. Tracing of the superior temporal gyrus benefited from standard neuroanatomical atlases, as was done in our previous neuroimaging studies on a variety of psychiatric disorders.12,13 Also, a method of Takahashi et al14 was used. According to their tracing method, the first coronal plane that demonstrated the temporal-frontal junction was accepted as the anterior boundary of the superior temporal gyrus. The other coronal plane consisting of the posterior end of the posterior horizontal limb of the Sylvian fissure was described as the posterior boundary of the whole superior temporal gyrus. Meanwhile, as defined by Takahashi et al,14 for each coronal slice, the Sylvian fissure superiorly bounded the whole superior temporal gyrus, while the superior temporal sulcus bounded inferiorly. The gyrus was then divided into supratemporal and lateral parts by using the lateral limb of the supratemporal plane. Meanwhile, the Heschl’s gyrus was traced from posterior to anterior, starting with the first slice consisting of the Heschl’s sulcus. The end slice for the Heschl’s gyrus was traced to the most anterior point of the Heschl’s sulcus or the sulcus intermedius if it appeared. Medial landmarks of the Heschl’s gyrus were the Sylvian fissure, inferior circular insular sulcus, or the first transverse sulcus, while the lateral landmark was the Heschl’s sulcus. Tracing of the Heschl’s gyrus was stopped, which was performed in a diagonal manner on the supratemporal plane; then, the areas anteromedial and posterolateral to the gyrus within the gray matter of the supratemporal plane were accepted as planum polare and planum temporale, respectively. Via the plane containing the anterior hip of the Heschl’s gyrus, the lateral superior temporal gyrus was divided into rostral and caudal parts. All tracings and volumetric measurements were performed by a senior radiologist who was blind to patients’ diagnoses and subject group. Additionally, we calculated intrarater and interrater intraclass correlation coefficients in 10 randomly selected subjects, which were ≥ 0.90 for all subregions of the superior temporal gyrus. Sample imaging belonging to the insula is presented in Figure 1. All results are presented in cubic millimeters.
Statistical Analysis
All statistical analyses were conducted using the SPSS version 22.0 (IBM, Armonk, New York). Statistical analyses were calculated with χ2 test, independent samples t test, ANCOVA, and Spearman correlation test. While the χ2 test was used to compare the categorical variables, the independent samples t test was utilized to compare continuous variables including volumes of the superior temporal gyrus. To control the effects of some variables such as age, sex, and total brain volumes, ANCOVA was preferred. When performing the correlation analyses, Spearman rank test was utilized. Statistical significance was accepted as P < .05 by a 2-tailed test.
RESULTS
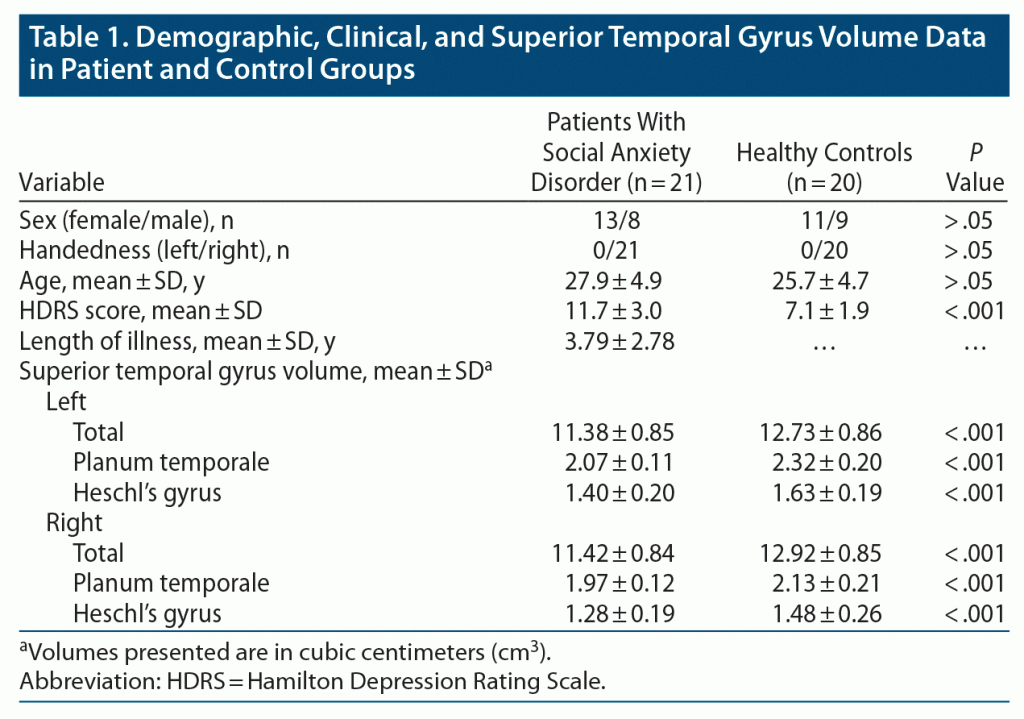
Demographic and clinical characteristics of the subjects are provided in Table 1. As seen in Table 1, there were no statistical differences between patients with a social anxiety disorder and healthy controls in regard to handedness, duration of education, sex distribution, or age (mean ± SD age = 30.89 ± 4.26 years for patients and 29.46 ± 6.12 years for controls).
For the volumetric data, we determined that the mean volume of superior temporal gyrus was statistically significantly smaller in patients than controls, as seen in Table 1 (left side: 11.38 ± 0.85 cm3 for patients and 12.73 ± 0.86 cm3 for controls, t = –5.064, P < .001; right side: 11.42 ± 0.84 mm3 for patients and 12.92 ± 0.85 cm3 for controls, t = –5.574, P < .001). Volumetric measurements of all subregions were also statistically significantly smaller in patients than in controls (Heschl’s gyrus left side: 1.40 ± 0.20 cm3 for patients and 1.63 ± 0.19 cm3 for controls, t = –3.679, P < .001; Heschl’s gyrus right side: 1.28 ± 0.19 cm3 for patients and 1.48 ± 0.26 cm3 for controls, t = –2.918, P < .01; planum temporale left side: 2.07 ± 0.11 cm3 for patients and 2.32 ± 0.20 cm3 for controls, t = –4.891, P < .001; planum temporale right side: 1.97 ± 0.12 cm3 for patients and 2.13 ± 0.21 cm3 for controls, t = –2.845, P < .01). In addition to absolute volumetric comparisons of the superior temporal gyrus subregion between patients with social anxiety disorder and controls, we also compared them using the ANCOVA undercontrolling for sex, age, and total brain volumes to exclude the effects of these parameters. We observed that reduced volumes for all of the superior temporal gyrus subregions were maintained (total superior temporal gyrus left side: P < .001, right side: P < .01; Heschl’s gyrus left side: P < .001, right side: P < .01; planum temporale left side: P < .001, right side: P < .01).
When correlational analyses were performed, Spearman correlation showed no significant correlation between the volumes of superior temporal gyrus and any demographic and clinical variables in both patients and controls (P > .05).
DISCUSSION
This is the first study, to our knowledge, investigating the superior temporal gyrus volumes in patients with a social anxiety disorder. Thus, we would like to emphasize the main findings. We determined that the mean volume of superior temporal gyrus was statistically significantly smaller in patients with social anxiety disorder than in healthy control subjects for both sides. Moreover, when comparing volumetric measurements for subregions, we detected that volumes of all subregions were also statistically significantly smaller in patients than in healthy controls (for both sides of the planum polare, Heschl’s gyrus, planum temporale, caudal superior temporal gyrus, and rostral superior and temporal gyrus). On the other hand, the ANCOVA undercontrolling for sex, age, and total brain volumes showed that reduced volumes for all of the superior temporal gyrus subregions were maintained. As mentioned previously, the superior temporal gyrus is 1 of 3 gyri in the temporal lobe of the brain and contains the important brain regions Brodmann’s areas 41 and 42, which are responsible for the sensation of sounds, and Wernicke’s area, which is responsible for the understanding of speech.8 But, more importantly and in association with a probable clinical picture of social anxiety disorder, the superior temporal gyrus is associated with auditory processing, including language, but also has been emphasized as a critical structure in social cognition.8 Furthermore, it has been noted that the superior temporal gyrus might be involved in the perception of emotions in facial stimuli, which could be associated with a social anxiety disorder.10 It has been suggested that the superior temporal gyrus may be involved in social intelligence.15 Moreover, some studies16,17 examining primates revealed that cells that function in the identification of facial expressions are located in the superior temporal gyrus. Also, a functional neuroimaging study by Baron-Cohen et al18 showed that the amygdala, superior temporal gyrus, and prefrontal cortex were stimulated during healthy subjects’ social performance. Thus, the the superior temporal gyrus seems to be linked to the neuroanatomy of social anxiety disorder. However, until now, no study has evaluated the volumes of superior temporal gyrus in patients with a social anxiety disorder.
De Bellis et al15 performed an MRI study to determine the superior temporal gyrus, thalamus, and prefrontal cortex volumes in 13 child and adolescent patients with generalized anxiety disorder and healthy controls. They found that total white matter and gray matter volumes of the superior temporal gyrus were statistically significantly larger in patients with generalized anxiety disorder than in those of healthy subjects, with a more pronounced right > left asymmetry in total and white matter volumes of superior temporal gyrus in the patient group and no volumetric differences of the thalamus and prefrontal cortex volumes. In another study, De Bellis et al19 examined children with maltreatment-related posttraumatic stress disorder and found that these patients had statistically significantly larger right, left, and total superior temporal gyrus and superior temporal gyrus gray volumes and findings of significant side-by-diagnosis interactions for superior temporal gyrus volumes, considering that there might be a more pronounced right > left asymmetry in total and posterior superior temporal gyrus volumes, with no differences in prefrontal, hippocampal, or basal ganglion structures. The findings in patients with generalized anxiety disorder and posttraumatic stress disorder seem to be contrary to our results. What can account for this difference? Social anxiety disorder has some discriminative features compared to other anxiety disorders with regard to neurobiological basis.20 For example, patients with social anxiety disorder have an undisturbed hypothalamic-pituitary-adrenal axis, which is contrary to dexamethasone suppression test results detected in patients with other anxiety disorders and depression.20 Moreover, patients with social anxiety disorder have normal hypothalamic-thyroid-adrenal axis, which is contrary to patients with other anxiety disorders and depression. On the other hand, compared to other anxiety disorders, in the pathophysiology of social anxiety disorder, dopamine seems to be more important than serotonin and norepinephrine. In this context, it can be speculated that our findings in patients with a social anxiety disorder may be implicating an important discriminative difference between other anxiety disorders.
However, our present study did not assess functionality, and structural neuroimaging findings may not accompany functional changes, but reduced volumes of superior temporal gyrus might be related to the neuroanatomic pathophysiology of social anxiety disorder. Given that this is the first study to evaluate superior temporal gyrus volumes in patients with social anxiety disorder, replication of the results by structural and functional neuroimaging investigations are needed.
Our study does have some limitations. First, the number of patients with social anxiety disorder and healthy controls was limited and should be increased in future studies. Second, we did not utilize any neuropsychological test to account for the functional counterpart of our structural data. Third, we did not exclude the presence of depressive disorder, which could have influenced our results. Fourth, although patients were receiving stable doses of psychopharmacologic agents, they were not treatment naive. Drug use can affect volumetric changes. Finally, we utilized the manual tracing method to measure the volumes of the superior temporal gyrus. As can be expected, the method itself might have affected our results.
Consequently, we suggest that patients with social anxiety disorder seem to have smaller superior temporal gyrus volumes compared to healthy control subjects, though we do not know whether these results meet functional changes of the same region. Thus, novel studies are required to structurally replicate our findings and to examine functional alterations in the superior temporal gyrus accompanying social cues.
Submitted: September 15, 2020; accepted December 29, 2020.
Published online: August 26, 2021.
Potential conflicts of interest: None.
Funding/support: This study was supported by Firat University Scientific Support Unit (FUBAP), Elazig, Turkey.
Role of the sponsor: The sponsor was involved in the design, conduct, and management of the study.
Clinical Points
- The superior temporal gyrus has been implicated as an important structure in social cognition processes.
- The mean volume for both sides of the superior temporal gyrus was statistically significantly smaller than those of healthy control subjects.
References (20)

- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. DSM-5 Diagnostic Classification. Washington, DC: American Psychiatric Association; 2013.
- Lurie SN, Doraiswamy PM, Husain MM, et al. In vivo assessment of pituitary gland volume with magnetic resonance imaging: the effect of age. J Clin Endocrinol Metab. 1990;71(2):505–508. PubMed CrossRef
- Takano K, Utsunomiya H, Ono H, et al. Normal development of the pituitary gland: assessment with three-dimensional MR volumetry. AJNR Am J Neuroradiol. 1999;20(2):312–315. PubMed
- Atmaca M. Pituitary gland in psychiatric disorders: a review of neuroimaging findings. Pituitary. 2014;17(4):392–397. PubMed CrossRef
- Potts NLS, Davidson JRT, Krishnan KR, et al. Magnetic resonance imaging in social phobia. Psychiatry Res. 1994;52(1):35–42. PubMed CrossRef
- Irle E, Ruhleder M, Lange C, et al. Reduced amygdalar and hippocampal size in adults with generalized social phobia. J Psychiatry Neurosci. 2010;35(2):126–131. PubMed CrossRef
- Koç M, Kuloğlu Ö, Yıldırım H, et al. The investigation of hippocampus and amygdala volume changes with MRI in patients with social anxiety disorder. Anatol J Psychiatry. 2018;19(2):150–156.
- Vander Ghinst M, Bourguignon M, Op de Beeck M, et al. Left superior temporal gyrus is coupled to attended speech in a cocktail-party auditory scene. J Neurosci. 2016;36(5):1596–1606. PubMed CrossRef
- Adolphs R. Is the human amygdala specialized for processing social information? Ann N Y Acad Sci. 2003;985(1):326–340. PubMed CrossRef
- Radua J, Phillips ML, Russell T, et al. Neural response to specific components of fearful faces in healthy and schizophrenic adults. Neuroimage. 2010;49(1):939–946. PubMed CrossRef
- First MB, Williams JBW, Spitzer RL, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT). New York: Biometrics Research, New York State Psychiatric Institute; 2007.
- Talairach J, Tournoux P. Co-Planar Stereotaxis Atlas of the Human Brain: 3-D Proportional System. New York, NY: Thieme Medical Publisher; 1988.
- Daniels DL, Haughton VM, Naidich TP. Cranial and Spinal Magnetic Resonance Imaging: An Atlas and Guide. New York, NY: Raven Press; 1987.
- Takahashi T, Wood SJ, Yung AR, et al. Superior temporal gyrus volume in antipsychotic-naive people at risk of psychosis. Br J Psychiatry. 2010;196(3):206–211. PubMed CrossRef
- De Bellis MD, Keshavan MS, Shifflett H, et al. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2002;51(7):553–562. PubMed CrossRef
- Desimone R. Face-selective cells in the temporal cortex of monkeys. J Cogn Neurosci. 1991;3(1):1–8. PubMed CrossRef
- Hasselmo ME, Rolls ET, Baylis GC. The role of expression and identity in the face-selective responses of neurons in the temporal visual cortex of the monkey. Behav Brain Res. 1989;32(3):203–218. PubMed CrossRef
- Baron-Cohen S, Ring HA, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11(6):1891–1898. PubMed CrossRef
- De Bellis MD, Hall J, Boring AM, et al. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50(4):305–309. PubMed CrossRef
- Martin EI, Ressler KJ, Binder E, et al. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North Am. 2009;32(3):549–575. PubMed CrossRef
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top