ABSTRACT
Pramipexole is a dopaminergic pharmacologic agent with reported adverse effects that include hypersexuality, shift in sexual interests, pathological gambling, compulsive shopping, and binge eating. Pramipexole is indicated in the treatment of Parkinson’s disease and restless leg syndrome and has been used as adjunctive or add-on treatment in major depressive disorder. This report describes the successful treatment of a series of 4 adult men who presented with concerns about problematic sexual interests and behaviors that began after treatment with pramipexole related to Parkinson’s disease or restless leg syndrome.
Prim Care Companion CNS Disord 2021;23(1):20br02663
To cite: Murphy L, Ly T, DiMario K, et al. Treatment of pramipexole-induced problematic sexual behaviors. Prim Care Companion CNS Disord. 2021;23(1):20br02663.
To share: https://doi.org/10.4088/PCC.20br02663
© Copyright 2021 Physicians Postgraduate Press, Inc.
aSexual Behaviours Clinic, Integrated Forensic Program, Royal Ottawa Mental Health Centre, Ottawa, Ontario, Canada
bDepartment of Psychology and Department of Criminology, University of Ottawa, Ottawa, Ontario, Canada
cDepartment of Psychiatry and Behavioural Neurosciences, McMaster University, Hamilton, Ontario, Canada
dDepartment of Psychiatry, University of Ottawa, Ottawa, Ontario, Canada
*Corresponding author: J. Paul Fedoroff, MD, Department of Psychiatry Chair, University of Ottawa, 1145 Carling Ave, Ottawa, Ontario, Canada K1Z 7K4 ([email protected]).
Pramipexole is a dopaminergic agonist most often used to treat symptoms of Parkinson’s disease (PD) and related movement disorders, including restless leg syndrome (RLS) and periodic limb movement disorder. Pramipexole is specifically used to improve motor activity, which in turn improves quality of life in patients diagnosed with PD and RLS.1
Unfortunately, pramipexole has been associated with adverse side effects including addictive and compulsive behaviors, hypersexuality, deviant sexual interests, pathological gambling, compulsive shopping, and binge eating.2–10 In many cases, the patient may not make the connection between using pramipexole and the emergence of compulsive interests and behaviors unless specifically asked by the prescribing physician, leading to underreporting of the actual frequency of these side effects.11 Primary care physicians who prescribe pramipexole for movement-related disorders should be aware of impulsive or disinhibited behaviors as a common side effect of pramipexole and work with patients to monitor and treat these problematic side effects.
In 2016, Public Citizen, a large consumer advocacy organization in the United States, submitted a petition to the US Food and Drug Administration to place black box warnings on 6 different products that are used in the treatment of PD.12 Black box warnings appear on the label of prescription medications to alert doctors and patients of potential serious adverse effects or life-threatening risks. Mirapex (pramipexole) was included on the list.12 The advocacy group suggested that, based on its review of more than 80 reports on the side effects of these medications, there is a “causal relationship” between these medications and impulsive behaviors. In the clinical trials reviewed, reported rates of the emergence of impulsive behaviors after administration of the medications ranged from 2.6% to 18.4%.13 The advisory group cautioned, however, that these rates are most likely conservative due to the potential for underreporting of impulsive behaviors and a failure to link the onset of the adverse effects to the medication.12
A review14 of a 2020 Canadian product monograph for Mirapex lists the development of impulsive behaviors as a potential adverse side effect. Although the listed symptoms include sexual problems, they are reported in a lengthy list of possible side effects and are not listed under the cautionary “Serious Warnings and Precautions” section.14 In contrast, the corresponding 2020 US product monograph15 for Mirapex does list “impulse control/compulsive behaviors” at the forefront of the document. It also provides references to case reports and cross-sectional research on the side effects of dopaminergic medications.15 However, these side effects are currently not formally noted as a black box warning in either Canada or the United States.
When sexual side effects are identified, the most common treatment involves a decrease or complete cessation of pramipexole.3,5,16,17 This can be problematic, as reduction of dopamine agonists is associated with a return of the problematic motor symptoms for which the pramipexole was originally prescribed.1 Alternative options to treat the impulsive interests, other than the discontinuation of pramipexole, include the use of cognitive-behavioral therapy18 or the administration of an opioid antagonist such as naltrexone.19 Of course, impulse control symptoms can develop independently of PD, RLS, or their treatments.20,21
The purpose of this case series is to describe the presentation and treatment of 4 men who presented with concerns about problematic sexual interests and behaviors that began after the commencement of treatment with pramipexole for PD or RLS. Three of the 4 men were able to continue using pramipexole to treat their movement disorders by separately treating their problematic sexual interests and behaviors. Depending on the patient, treatment consisted of adjustment, maintenance, or cessation of pramipexole along with the voluntary addition of an antiandrogen intended to decrease the person’s sex drive and manage the sexual side effects. Here, we include terms such as hypersexuality, sexual addiction, and sexual compulsion because they are terms the literature or patients used and not because the authors of this report necessarily endorse them.
METHODS
Participants
All patients in this case series were referred to the Sexual Behaviours Clinic (SBC) in Ottawa, Canada for assessment and treatment of problematic sexual interests and behaviors. Prior to assessment, all patients consented to use of their anonymized data for publication and signed an ethically approved consent form. Four patients who had been referred with symptoms of problematic sexual interests and behaviors after initiating treatment with pramipexole (Mirapex) were included in this case series.
Procedure
The study design is a retrospective review based on medical records collected from the SBC at the Royal Ottawa Mental Health Centre in Ottawa, Canada. Numerous ethically approved retrospective studies have been conducted using patient files from the SBC. Approval was obtained from the appropriate institutional ethics review board. Comparisons of outcomes in the 4 cases of men experiencing sexual side effects from pramipexole were conducted. Materials included demographic information such as age, marital status, employment, sexual orientation, and criminal and psychiatric history. Medication history was also recorded including previous and current medication type, dose, and timeline of use when available. Self-report measures and collateral reports were taken from clinical charts detailing experiences while taking pramipexole. The authors also investigated common themes in the clinical experiences, medication use, and treatment among the 4 study participants. Information prior to and after changes in medication type and dose was reviewed. Outcomes including the possible impact of antiandrogens on pramipexole-induced problematic sexual interests and behaviors are explored.
CASE DESCRIPTIONS
Case 1. Mr A, a 60-year-old man, worked as a public servant. He was referred for concerns about a significant increase in time spent viewing pornography and his self-reported obsessive sexual thoughts. He was diagnosed with PD in 2010. Following his diagnosis, he was prescribed pramipexole 1 mg administered 3 times daily. Approximately 5 months after starting the medication, he noted changes in his interests and behaviors. He reported the following:
I have an obsession with going to strip clubs, hiring escorts for sex, and online porn. It’s irrational behavior—doing things I never would have done [before the medication]. I spend money on sex and viewing sex, have impulsive behaviors, and would not be able to say no to irrational spending. From my life savings, I spent about $100,000 in just 1 year on sex. These [actions] have caused significant problems with my wife. The effect of Mirapex crept up on me and possessed me. After starting the medication, I became completely irrational and completely focused on sex to the exclusion of everything I once cared for and enjoyed—I am obsessed.
He first became aware of his problem approximately 2 years before seeking treatment for his sexual preoccupations and behaviors. He reported that he sought treatment because “My behavior resulted in my marriage breakdown. I feel guilty and disappointed in myself. I am depressed about my future and prospects for happiness.” He saw his main sexual problem to be associated with his “obsessive behavior.” He identified the cause of his problem as “taking Mirapex for my Parkinson’s disease.” Mr A reported no interest in other addictive behaviors reportedly associated with pramipexole, such as gambling. Mr A explained the consequences of his actions as follows:
My daughter won’t talk to me; my wife is angry, hurt, and disillusioned. She has developed her own mental health issues. I didn’t have any feelings of guilt [about my behaviors] early on, but as time went on, I felt ashamed. Now, I have full-blown guilt and regret. I spent my life savings . . . this altered my retirement plans with my wife. My wife and I are now separated. I did not notice anything until 5 months after starting Mirapex. I became obsessed with going to strip clubs and became obsessed with a stripper. I never had sex with her. Then, I became obsessed with another stripper and had sex with her over 40 times for which I paid $500 every time. I hired escorts regularly.
Approximately 2 years after Mr A’s PD diagnosis, his dose of pramipexole was tapered from 1 mg to 0.5 mg in the hope of decreasing his interests and behaviors. He reported some decrease in his problematic interests but still had concerns. He said “I noticed a difference in the intensity of my obsessions in the last month but I still have them.” Two months after the initial tapering, pramipexole was stopped completely. He reported a progressive decrease in his problematic sexual interests. Three months after pramipexole was discontinued, Mr A reported no sexual concerns, explaining “I do not have the sexual impulses I had on Mirapex. I have no interest in illicit sex. I am now the person I was before [I was on] Mirapex. When I was on Mirapex, I lost consideration for the consequences [of my actions].”
As expected, after discontinuation of pramipexole, Mr A reported increased difficulty with dyskinesia and other motor symptoms related to PD. He was prescribed levodopa 25 mg 3 times daily, which was effective in relieving his motor symptoms. On this medication regimen, he reported no further sexual preoccupations. The use of levodopa to treat patients with PD without inducing sexual side effects has been described elsewhere.22
Case 2. Mr B, a 50-year-old man, worked as a computer programmer but was on disability due to symptoms of PD. After his PD was diagnosed, he was treated with pramipexole 1.5 mg 3 times daily. He was later referred for assessment due to concerns about addictive and compulsive sexual behaviors. After being treated with pramipexole, he reported:
Mirapex has turned on my “horniness light.” It takes away my guilt. I cannot stop my sexual behaviors. I have sex with people I don’t know, sometimes more than once per day, and sometimes with more than 1 person per day. This problem started for me 3 years ago and has gotten worse over time. I go out at night for sex. I tell my wife I am going gambling. Sometimes instead of sex I went gambling, and now I am also addicted to gambling. I have lost a total of $5,000 while gambling over the past year. I feel like I am destroying my relationship [with my wife]. I want to kill myself. I would like to be a different person. I can’t stop looking for sex. I will screw anyone.
After being assessed in the SBC, Mr B’s dose of pramipexole was reduced to 0.5 mg 3 times daily. Subsequently, he stopped gambling and noted some decrease in his sexual preoccupations. His symptoms of PD continued to be well controlled. He was then prescribed oral medroxyprogesterone acetate (MPA) (Provera) 100 mg 3 times daily. At this dose his compulsive sexual interests were nearly in remission. Mr B’s MPA 100-mg dose was increased to 4 times daily. Approximately 1 month after this increase, Mr B reported a complete remission of unwanted sexual interests and behaviors. He commented, “I believe that being on Provera makes it so I can still be on Mirapex.” Unfortunately, there was no improvement in his relationship with his wife after the successful treatment of his sexual side effects.
Case 3. Mr C is a 72-year-old retired man. He was referred to the SBC after being charged with 2 counts of sexual assault. The first sexual assault charge involved overpowering a 71-year-old woman in a parking lot and forcing vaginal intercourse in her parked car. He left the scene without being arrested. Four days after the first assault, Mr C committed a second sexual assault on a woman in her mid-20s, who he followed after seeing her buy some lingerie. During the assault, he grabbed her purse, asking for the underwear in her purse. Mr C was found not criminally responsible on account of mental disorder for both offenses due to a diagnosis of frontal lobe vascular dementia. For both offenses, Mr C reported that his actions were motivated by urges to see the ladies’ undergarments or lingerie.
Upon initial assessment, Mr C reported that he had a “fetish for women’s clothes” since he was 14 years of age. He explained that he “would put slips on and masturbate.” He reported that for most of his life the interest did not cause him any concern, and he never even considered committing an offense to obtain lingerie. Mr C’s transvestic sexual interest was known to his wife, who tolerated the behavior throughout their marriage, and his interest was not considered a problem for them.
However, approximately 10 years prior to the index offense, at the age of 62 years, Mr C was diagnosed with RLS, for which he was prescribed pramipexole 1 mg 3 times daily. Mr C explained that the pramipexole “made a huge difference in my [RLS] symptoms. Without [Mirapex], I would be jumping up and down like a yo-yo.” Mr C maintained the same dose of pramipexole since he began treatment for his RLS.
However, despite the efficacy of pramipexole in managing his RLS symptoms, Mr C reported a significant increase in his interest in wearing women’s lingerie that coincided with his initial prescription of the medication. He explained that his interest became “almost obsessive” and he would do “anything” to get access to women’s lingerie. He disclosed that during this period he began stealing women’s lingerie or underwear from clotheslines in his neighborhood. He was never arrested or charged for those actions. He reported “When I see women, I immediately think about what they wear under their clothes. That’s my sickness. It’s slips that I like most.” Ten years after initially starting pramipexole, his behaviors escalated to the point that he committed the 2 sexual assaults for which he was later found not criminally responsible on account of mental disorder.
To alleviate his sexual concerns while continuing to treat RLS, he was prescribed MPA 100 mg once daily. Mr C subsequently reported a change in his sexual interests in women’s lingerie. He explained, “I still like to wear women’s lingerie under my clothes, but it is not sexual . . . just having my lingerie is enough. I don’t need other people’s lingerie. The MPA makes it so I do not go back to where I was . . . so I don’t hurt people.” Concurrent treatment with MPA allowed Mr C to continue treatment with pramipexole for his RLS by reducing the strength of his sex drive. This treatment did not change his sexual interests but reduced his need to act on them.
Case 4. Mr D, a 54-year-old unemployed man, suffers from RLS. After being diagnosed with RLS, he was prescribed pramipexole 0.125 mg 3 times daily and reported improved sleep. In his opinion, “pramipexole is a miracle drug.” Mr D was first assessed at the SBC before his trial for charges of accessing and possessing child pornography depicting girls (aged 11 to 17 years). He also had a large collection of pornography depicting women (aged 18 to 70 years). He denied ever masturbating while watching child pornography but reported having a “compulsive need to view pornography.” Despite improvement in his symptoms of RLS while taking pramipexole, he also reported problematic interest in internet pornography and an emergence of “an abnormal interest in teenagers and preteens.” Consequently, a medication trial with MPA was initiated while continuing treatment with pramipexole. He was prescribed MPA up to 400 mg daily and reported complete remission of problematic sexual urges and behaviors. The combined use of pramipexole and MPA allowed Mr D to adequately manage his RLS and problematic sexual interests.
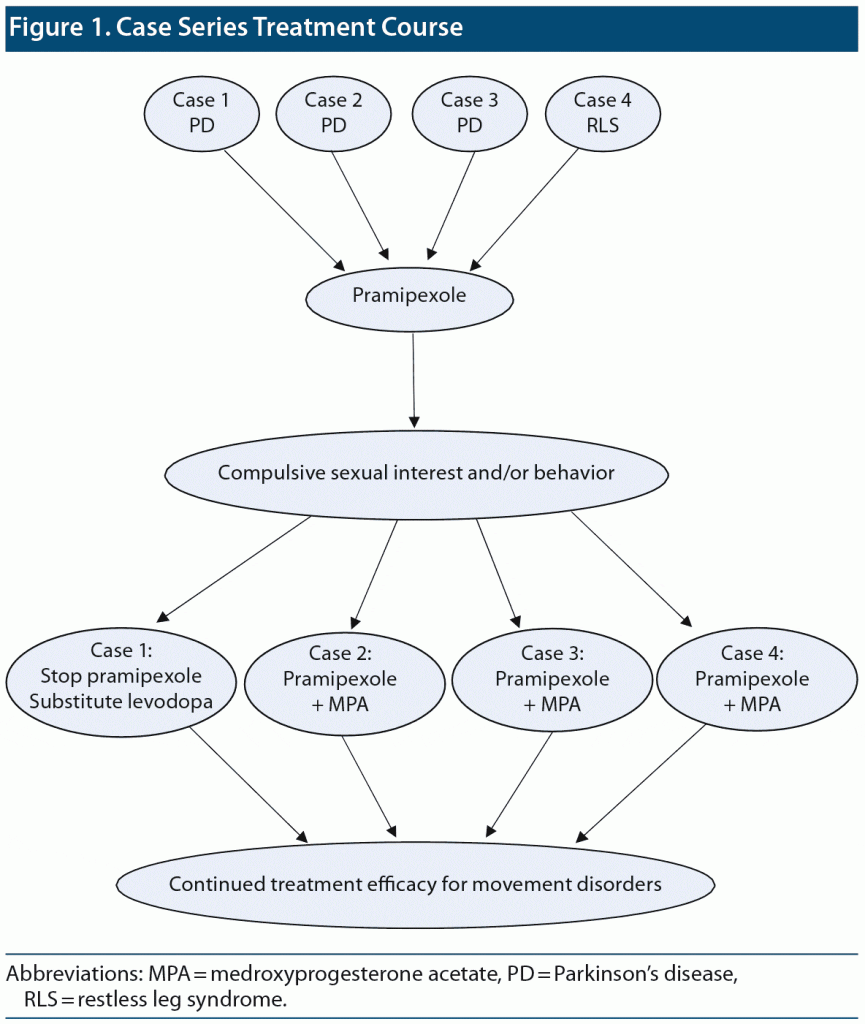
Figure 1 summarizes the course of treatment in the previously described cases.
DISCUSSION
Pramipexole is a nonergot direct dopamine agonist. Its mechanism of action in relation to treating PD is thought to be through its role as a dopamine receptor agonist, especially on specific D3 receptors in the striatum.23 Dopaminergic activity in the mesolimbic or reward pathway is associated with reports of compulsive behaviors that include compulsive eating, gambling, spending, and sex.22 The 4 men in this case series reported impulsive sexual behaviors and increased problematic sexual interests during treatment with pramipexole.
Complex repetitive behaviors such as impulse control disorders (ICDs) and hedonistic dysregulation have been reported.24 Other case studies have reported increased compulsive sexual behavior after using pramipexole,25,26 as well as worsening of these symptoms when increasing the dose of pramipexole.14 Research findings have questioned the association among problematic sexual interests and behaviors and dopamine agonist use. Physicians should be vigilant in monitoring for problematic sexual behaviors in all PD patients to whom they prescribe pramipexole.17,27 It is also important for physicians to remember that problematic sexual behaviors can affect not only the patient but also their family and friends.
Sansone and Ferlan26 recommended caution when initiating pramipexole in people with past sexual offenses due to concerns about compulsive masturbation and hypersexuality. A functional magnetic resonance imaging (fMRI) study conducted by Politis et al28 found that PD patients diagnosed with hypersexuality were more stimulated by sexual visual cues compared to PD patients without hypersexuality. Analysis of fMRI results also revealed that dopaminergic drugs may play a role in inhibiting local neuronal circuits in the cerebral cortex, which could result in an increased risk of impulsive sexual behaviors in this population.28 Future research could elaborate on other potential mechanisms of individual susceptibility to developing these behaviors with dopamine replacement therapy in patients with PD. Implications for predicting these adverse effects and providing alternative management strategies without discontinuing an efficacious pharmaceutical agent for symptoms of PD should also be explored further.
For individuals who may report the emergence of problematic sexual interests or behaviors after beginning pramipexole, questions arise regarding whether the sexual interests were present at baseline and exacerbated by pramipexole treatment or if the onset of problematic interests occurred directly as a result of treatment initiation and dose escalation with pramipexole.
For cases 1 and 2, it seems that the medication side effects included sexual preoccupations and behaviors regarding adult sexual activities that led to repeated extramarital affairs, attendance at strip clubs, and hiring of sex workers. On the basis of their reports, it seems that both men had nonconcerning interests in their adult partners prior to the use of pramipexole; however, the medication was associated with more persistent sexual preoccupations and behaviors. For case 3, treatment with pramipexole was associated with an exacerbation of a preexisting sexual interest in women’s lingerie that subsequently led to criminal activity to gain access to lingerie, including sexual offenses against 2 women that he said were directly due to his interest in their lingerie. Another independent case report29 has highlighted similar preexisting paraphilic interests that were exacerbated by pramipexole. In case 4, use of pramipexole coincided with emergence of a new sexual interest in pornography depicting minors.
Quinn et al30 observed that previously latent or underlying sadomasochistic interests were unmasked after initiating treatment with dopamine agonists for 2 of their patients with PD. After reducing the dose of dopaminergic agents, the sadomasochistic traits decreased.30 In contrast, Cannas et al31 indicated that their patient had no paraphilic fantasies prior to the PD diagnosis and dopaminergic treatment. However, Cannas et al31 did agree with Wilson7 that dopaminergic drugs could have unmasked prior paraphilic fantasies and facilitated their practice through disinhibition. Also, Cannas et al31 postulate the threshold of disinhibition of actions for the paraphilias may vary among individuals. Future studies may further explore this phenomenon and investigate the relationship between apparently new-onset problematic sexual interests and disinhibition or unmasking of previous interests and related compulsive sexual interests and behavior.
Dopamine dysregulation syndrome (DDS) involves a pattern of behavior that can occur when dopaminergic drugs are used to treat PD.24 These behavior patterns are sometimes referred to as ICDs and can include substance abuse, pathological gambling, compulsive sexual behaviors, and binge eating. They are frequently hidden and are often explained as an individual’s own behavioral repertoire, commonly experienced without subjective distress. These ICDs are more prevalent in persons with PD than in the general population (0.25%–3%). In individuals with PD treated with dopaminergic medications, this prevalence rises to between 14% and 17%. It is greater than the 0.7% prevalence reported in PD patients treated with levodopa alone.32 Dopaminergic agents used to treat PD reportedly increase the risk of developing ICD by 2- to 3-fold; 80% of these cases were found to occur in the first year of initiation of treatment with direct dopaminergic agonist medications.32
In the present case series, the dopaminergic medication, pramipexole, was related to onset or worsening of sexual interests and behaviors. However, other risk factors for compulsive behaviors in relation to PD may have also been present. Cilia et al24 found that patients with ICD or DDS are more likely to have a personal history of depressive symptoms or history of drug abuse. Consistent with this finding, all patients included in the present case study had a history of mood disorders, including depressive symptoms, and a personal or family history of drug or alcohol abuse. Cilia et al24 propose that the risk of DDS may also be genetically linked. Thus, a genetic association could explain the variation in the threshold of disinhibition among individuals.
Research has shown that sleep deprivation is associated with increased dopamine levels.33 Sleep deprivation also lowers inhibition and increases impulsivity.34 Increased dopamine levels may also increase impulsivity associated with obtaining immediate rewards.35 Since dopamine-enhancing drugs can increase wakefulness,33 perhaps individuals with previous sleep problems are further impacted by the sleep deprivation effects of dopaminergic treatment. It is possible that this sleep deprivation effect and the increased dopamine levels could make these individuals more susceptible to a disinhibition effect and increased impulsivity.
In this case series, 3 of the 4 men (cases 1, 2, and 4) had difficulty with insomnia and were diagnosed with sleep disorders. These patients experienced difficulty with sleep before initiation of pramipexole. In contrast, Mr C in case 3 had insomnia that was treated to some extent with trazodone and thus may have been less likely to be negatively affected by sleep deprivation.
The effects of sleep deprivation as well as the increased dopamine levels from dopaminergic agonist administration may have predisposed these individuals to problematic repetitive-reward behaviors via a disinhibition effect. Pramipexole use has also been associated with cognitive impairment, even in healthy individuals.36 This finding may explain the association between pramipexole treatment and problematic sexual behaviors, especially when PD and other risk factors related to developing DDS coexist.
Antiandrogen medications that prevent androgens from inducing activity on androgen target sites37 have been shown to decrease sexual desire and activity38 and are commonly used to treat paraphilic sexual disorders.37,39 Generally, the mechanism of action of antiandrogens in treating various sexual disorders in men is by decreasing serum testosterone.37 Although there is research on antiandrogen use to treat problematic sexual disorders,39 research on how antiandrogens may influence the dopaminergic system is sparse. Increased dopaminergic activity in the tuberoinfundibular tract inhibits prolactin production and release, resulting in stimulation of the Leydig cells in the testes to produce and release serum testosterone. Hyperprolactinemia is associated with low dopamine states or tuberoinfundibular tract dysfunction and has been associated with erectile dysfunction in males.40
Vadász et al41 found that cyproterone, an antiandrogen and progestin agonist, significantly decreased activity of tyrosine hydroxylase, thus suppressing dopaminergic activity in the mesencephalon. Tyrosine hydroxylase is the enzyme that catalyzes L-tyrosine into levodopa, which is the precursor for dopamine.42 Decreased activity in the dopaminergic pathways due to use of antiandrogens may explain the effect of antiandrogens on pramipexole-induced sexual interests and behaviors. Perhaps antiandrogens counteract the effects of increased dopamine levels in specific target sites of dopaminergic pathways, including those relating to sexual desire, arousal, interest, and behaviors. Further research is needed to examine the relationship between antiandrogens and dopamine pathways as they pertain to sexual interests and behaviors.
CONCLUSIONS
Prescription of pramipexole to treat PD or RLS for the 4 men in this case series was associated with an increased desire for sexual stimulation. A reduction or elimination of pramipexole dosage and/or the addition of antiandrogen medications permitted ongoing treatment of PD and RLS with pramipexole while successfully treating problematic sexual interests and behaviors. These observations support the hypothesis that pramipexole-associated sexual desire, arousal, and sex-seeking behavior may be effectively treated with testosterone-blocking agents.
Current studies have yet to fully explore the efficacy of antiandrogens to alleviate impulsive sexual behaviors and disinhibition, whose onset or worsening is associated with treatment with dopaminergic agonists such as pramipexole. In this case series, antiandrogens alleviated problematic sexual thoughts and behaviors. Given the limited sample size, it is difficult to generalize these findings. Also, other factors such as marital discord, unrelated mental health concerns, and medical conditions and their treatment may have also played a part in their presentation. However, this series may be taken as supportive evidence that antiandrogens can be used together with pramipexole and that they may assist men who experience problematic sexual interests or behaviors secondary to pramipexole. This may be an important intervention in men who require pramipexole to treat movement disorders but suffer from the adverse side effects of changes in sexual interests and behaviors.
Published online: February 18, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Previous presentation: This work was presented in part at the Canadian Academy of Psychiatry and the Law Annual Meeting; March 1–6, 2014; Lake Louise, Alberta, Canada, and the International Association of Sex Research Annual Meeting; August 4–7, 2013; Chicago, Illinois.
Patient consent: Consent was received from the patients to publish the case reports, and information has been de-identified to protect anonymity.
References (42)

- Fedorova NV, Chigir’ IP. Use of the dopamine receptor agonist Mirapex in the treatment of Parkinson’s disease. Neurosci Behav Physiol. 2007;37(6):539–546. PubMed CrossRef NLM
- Aiken CB. Pramipexole in psychiatry: a systematic review of the literature. J Clin Psychiatry. 2007;68(8):1230–1236. PubMed CrossRef NLM
- Bostwick JM, Hecksel KA, Stevens SR, et al. Frequency of new-onset pathologic compulsive gambling or hypersexuality after drug treatment of idiopathic Parkinson disease. Mayo Clin Proc. 2009;84(4):310–316. PubMed CrossRef NLM
- Claassen DO, van den Wildenberg WPM, Ridderinkhof KR, et al. The risky business of dopamine agonists in Parkinson disease and impulse control disorders. Behav Neurosci. 2011;125(4):492–500. PubMed CrossRef NLM
- Klos KJ, Bower JH, Josephs KA, et al. Pathological hypersexuality predominantly linked to adjuvant dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord. 2005;11(6):381–386. PubMed CrossRef NLM
- Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63(7):969–973. PubMed CrossRef NLM
- Wilson A. Disinhibition and narrative in the paraphilias. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(2):431. PubMed CrossRef NLM
- Garcia-Ruiz PJ. Impulse control disorders and dopamine-related creativity: pathogenesis and mechanism, short review, and hypothesis. Front Neurol. 2018;9:1041. PubMed CrossRef NLM
- Latt MD, Lewis S, Zekry O, et al. Factors to consider in the selection of dopamine agonists for older persons with Parkinson’s disease. Drugs Aging. 2019;36(3):189–202. PubMed CrossRef NLM
- Jeon N, Bortolato M. What drugs modify the risk of iatrogenic impulse-control disorders in Parkinson’s disease? a preliminary pharmacoepidemiologic study. PLoS One. 2020;15(1):e0227128. PubMed CrossRef NLM
- Hassan A, Bower JH, Kumar N, et al. Dopamine agonist-triggered pathological behaviors: surveillance in the PD clinic reveals high frequencies. Parkinsonism Relat Disord. 2011;17(4):260–264. PubMed CrossRef NLM
- Powell VD, Sorscher S, Carome M; Public Citizen’s Health Research Group. Petition to the FDA on Dopamine Agonist Drugs. Public Citizen website. https://www.citizen.org/wp-content/uploads/migration/2328.pdf. June 29, 2016.
- Silverman E. FDA urged to place warnings on Parkinson’s drugs about compulsive behaviors. STAT website. https://www.statnews.com/pharmalot/2016/06/29/fda-parkinsons-compulsive-behaviors/. June 29, 2016.
- Mirapex Product monograph. Boehringer-Ingelheim website. https://www.boehringer-ingelheim.ca/sites/ca/files/documents/mirapexpmen.pdf. March 2020.
- Mirapex (pramipexole dihydrochloride) Prescribing Information. Boehringer-Ingelheim website. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Mirapex/Mirapex.pdf. January 2020.
- Fawcett J, Rush AJ, Vukelich J, et al. Clinical experience with high-dosage pramipexole in patients with treatment-resistant depressive episodes in unipolar and bipolar depression. Am J Psychiatry. 2016;173(2):107–111. PubMed CrossRef NLM
- Rocha FL, Hara C. Compulsive masturbation with pramipexole for antidepressant augmentation in major depression: a case report. J Clin Psychopharmacol. 2015;35(4):484–485. PubMed NLM
- Okai D, Askey-Jones S, Samuel M, et al. Trial of CBT for impulse control behaviors affecting Parkinson patients and their caregivers. Neurology. 2013;80(9):792–799. PubMed CrossRef NLM
- Haussermann P, Goecker D, Beier K, et al. Low-dose cyproterone acetate treatment of sexual acting out in men with dementia. Int Psychogeriatr. 2003;15(2):181–186. PubMed CrossRef NLM
- Driver-Dunckley ED, Noble BN, Hentz JG, et al. Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clin Neuropharmacol. 2007;30(5):249–255. PubMed CrossRef NLM
- Weintraub D, Papay K, Siderowf A; Parkinson’s Progression Markers Initiative. Screening for impulse control symptoms in patients with de novo Parkinson disease: a case-control study. Neurology. 2013;80(2):176–180. PubMed CrossRef NLM
- Ahlskog JE. Pathological behaviors provoked by dopamine agonist therapy of Parkinson’s disease. Physiol Behav. 2011;104(1):168–172. PubMed CrossRef NLM
- Seeman P. Parkinson’s disease treatment may cause impulse-control disorder via dopamine D3 receptors. Synapse. 2015;69(4):183–189. PubMed CrossRef NLM
- Cilia R, Siri C, Canesi M, et al. Dopamine dysregulation syndrome in Parkinson’s disease: from clinical and neuropsychological characterisation to management and long-term outcome. J Neurol Neurosurg Psychiatry. 2014;85(3):311–318. PubMed CrossRef NLM
- Munhoz RP, Fabiani G, Becker N, et al. Increased frequency and range of sexual behavior in a patient with Parkinson’s disease after use of pramipexole: a case report. J Sex Med. 2009;6(4):1177–1180. PubMed CrossRef NLM
- Sansone RA, Ferlan M. Pramipexole and compulsive masturbation. Psychiatry (Edgmont). 2007;4(9):57–59. PubMed NLM
- Cooper CA, Jadidian A, Paggi M, et al. Prevalence of hypersexual behavior in Parkinson’s disease patients: not restricted to males and dopamine agonist use. Int J Gen Med. 2009;2:57–61. PubMed CrossRef NLM
- Politis M, Loane C, Wu K, et al. Neural response to visual sexual cues in dopamine treatment-linked hypersexuality in Parkinson’s disease. Brain. 2013;136(pt 2):400–411. PubMed CrossRef NLM
- Tajima-Pozo K, Bardudo E, Aguilar-Shea AL, et al. Pramipexole: paraphilia as adverse drug reaction. J Neurol Neurosci. 2010;2(1):3.
- Quinn NP, Toone B, Lang AE, et al. Dopa dose-dependent sexual deviation. Br J Psychiatry. 1983;142(3):296–298. PubMed CrossRef NLM
- Cannas A, Solla P, Floris GL, et al. Dopaminergic drugs, paraphilic fantasies, paraphilic behaviours and creativity in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(3):563–564. PubMed CrossRef NLM
- Voon V, Hassan K, Zurowski M, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67(7):1254–1257. PubMed CrossRef NLM
- Volkow ND, Wang GJ, Telang F, et al. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28(34):8454–8461. PubMed CrossRef NLM
- Anderson C, Platten CR. Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behav Brain Res. 2011;217(2):463–466. PubMed CrossRef NLM
- Pine A, Shiner T, Seymour B, et al. Dopamine, time, and impulsivity in humans. J Neurosci. 2010;30(26):8888–8896. PubMed CrossRef NLM
- Hamidovic A, Kang UJ, de Wit H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. J Clin Psychopharmacol. 2008;28(1):45–51. PubMed CrossRef NLM
- Neumann F, Töpert M. Pharmacology of antiandrogens. J Steroid Biochem. 1986;25(5B):885–895. PubMed CrossRef NLM
- Bancroft J, Tennent G, Loucas K, et al. The control of deviant sexual behaviour by drugs, I: behavioural changes following oestrogens and anti-androgens. Br J Psychiatry. 1974;125(0):310–315. PubMed CrossRef NLM
- Murphy L, Bradford J, Fedoroff JP. (2014). Treatment of paraphilias and paraphilic disorders. In: Gabbard GO, ed. Gabbard’s Treatment of Psychiatric Disorders. 5th Ed. Arlington, VA: American Psychiatric Publishing; 669–694.
- Zeitlin SI, Rajfer J. Hyperprolactinemia and erectile dysfunction. Rev Urol. 2000;2(1):39–42. PubMed NLM
- Vadász C, Kobor G, Kabai P, et al. Perinatal anti-androgen treatment and genotype affect the mesotelencephalic dopamine system and behavior in mice. Horm Behav. 1988;22(4):528–539. PubMed CrossRef NLM
- Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508(1):1–12. PubMed CrossRef NLM
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top