Ritonavir/Lopinavir and Its Potential Interactions With Psychiatric Medications:
A COVID-19 Perspective
The coronavirus disease 2019 (COVID-19) pandemic is a global catastrophe. One of the medications being proposed for the treatment of COVID-19 is the antiretroviral drug combination of ritonavir and lopinavir.1 Ritonavir is a potent cytochrome P450 (CYP) 2D6, 3A4, and 1A2 inhibitor and a CYP2B6, CYP2C19, and glucuronidation inducer.1 It is also an inducer and inhibitor of p-glycoprotein (a transmembrane drug efflux pump that extrudes toxins and hence protects organs).2 It is also a CYP2D6 and CYP3A4 substrate. CYP inhibition of ritonavir rises with increased dose, and it affects CYP2D6 more than other CYP enzymes. Lopinavir is primarily metabolized by CYP3A4. Lopinavir, when combined with a low dose of ritonavir, inhibits its own metabolism and thus augments its effect.3 Psychotropics are metabolized by the CYP enzymes and have a multitude of drug interactions with this antiretroviral combination.1
Antidepressants
Selective serotonin reuptake inhibitors. Citalopram and escitalopram are substrates of CYP2C19 and CYP3A44 and also weakly inhibit and are a substrate of CYP2D6. However, escitalopram combined with ritonavir was not found to produce clinically relevant changes.4,5 Escitalopram and citalopram are metabolized through multiple different pathways and so have less propensity for interactions. Thus, escitalopram and citalopram are preferred over other antidepressants due to minimal interactions with ritonavir.1,6
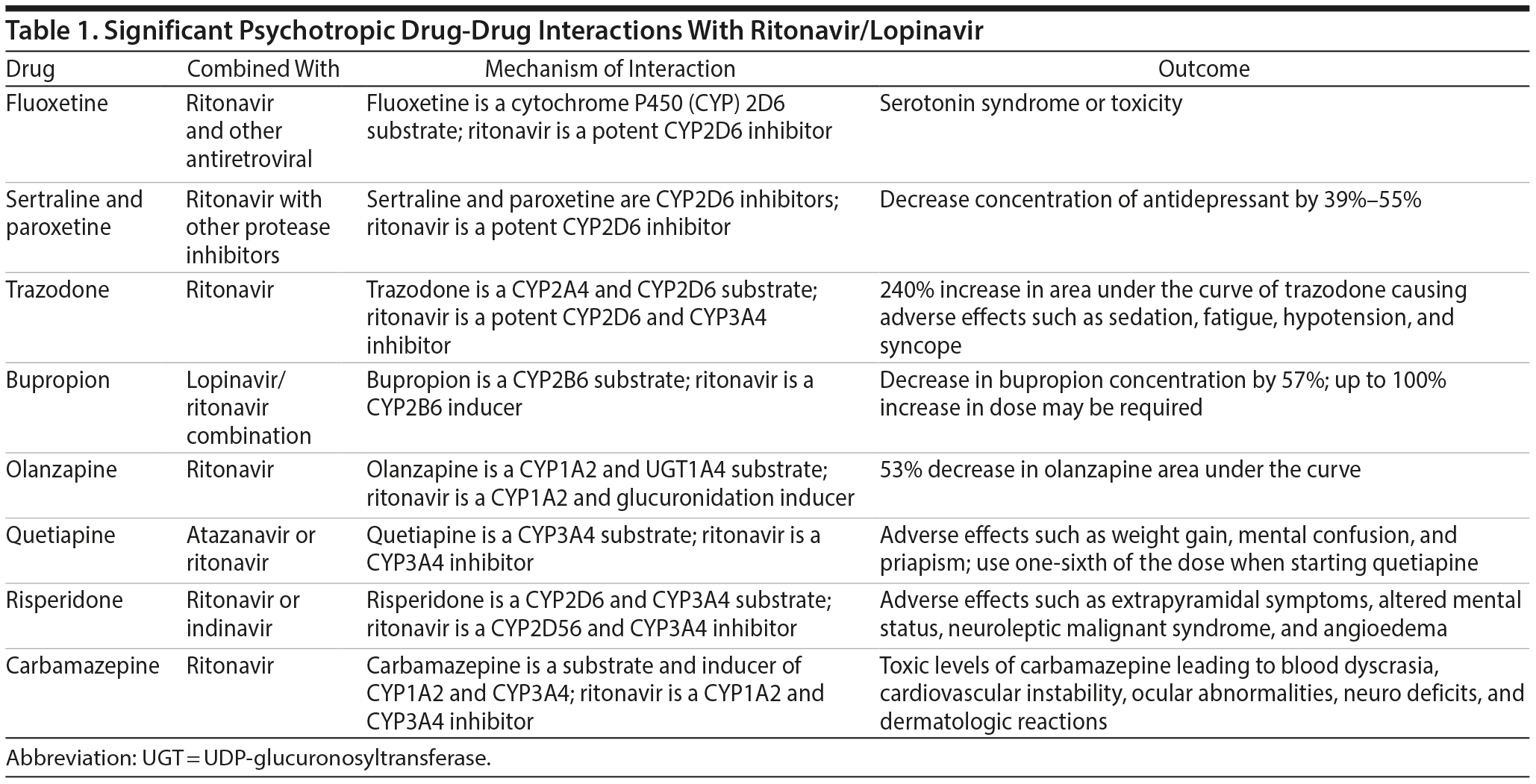
Fluoxetine is predominantly metabolized through CYP2D6 and CYP3A4. It also has inhibitory effects on CYP2D6, CYP2C9, CYP2C19, and CYP3A4, with the most potent effect on CYP2D6. When coadministered with a high dose of ritonavir (400-1200 mg), fluoxetine has been reported to cause serotonin syndrome or serotonin toxicity and should be used with caution.1,6,7
Fluvoxamine is metabolized through CYP2D6 and CYP1A2 and has potent inhibitory effects on CYP1A2 and CYP2C19, moderate inhibitory effects on CYP2C9 and CYP3A4, and weak inhibitory effects on CYP2D6. When coadministered with protease inhibitors (PIs), the concentration of the PIs could potentially increase or decrease given the effects on multiple CYP enzymes, so another antidepressant drug is recommended.6
Serotonin-norepinephrine reuptake inhibitors. Venlafaxine is a substrate and weak inhibitor of CYP3A4 and CYP2D6, as is desvenlafaxine. There is a paucity of literature regarding venlafaxine coadministered with ritonavir or lopinavir. It is recommended that extended-release formulations of venlafaxine and desvenlafaxine be used if the benefits exceed the risks, as research on venlafaxine with indinavir, a similar drug, showed the same results.1,6
Duloxetine is metabolized by CYP2D6 and CYP1A2. When combined with a potent CYP2D6 or CYP1A2 inhibitor, duloxetine’s concentrations increased significantly.3,6
Paroxetine, a CYP2D6 inhibitor, increases the concentration of duloxetine by 60%, and CYP1A2 inhibitors increase the level of duloxetine by 6-fold.6 Such interaction could be possible with other potent CYP2D6 inhibitors such as ritonavir. Ritonavir in a dose of 200 mg twice/day when coadministered with duloxetine has caused adverse effects such as sedation, fatigue, hypotension, and syncope potentially due to increased levels.6 For this reason, an alternative antidepressant should be considered when coadministered with ritonavir.6
Milnacipran and levomilnacipran are metabolized minimally by CYP3A4 and require dose reduction when coadministered with potent CYP3A4 inhibitors like ritonavir.1,6 However, literature regarding these interactions is sparse.
Tricyclic antidepressants. These antidepressants are substrates of CYP3A4 and CYP2D6. When tricyclic antidepressants are used with a CYP2D6 inhibitor such as ritonavir, close monitoring is recommended.1
Monoamine oxidase inhibitors. Monoamine oxidase inhibitors do not potentially interact with ritonavir with regard to CYP enzymes. However, we do not recommend using a monoamine oxidase inhibitor with ritonavir due to its narrow therapeutic index and potential cardiac side effects.1
Miscellaneous. Trazodone is metabolized by CYP2D6 and CYP3A4. The levels of trazodone can be significantly increased when used with ritonavir, theoretically up to 240%, with an increase in side effects such as sedation, hypotension, and syncope.1,6 If trazodone is indicated, it should be started at a low dose and monitored for adverse effects.
Mirtazapine is metabolized by CYP1A2, CYP3A4, and CYP2D6 with no appreciable inhibitory or inducing properties.1,3,6 There are no well-delineated drug interactions between mirtazapine and antiretrovirals.
Bupropion is a substrate of CYP2B6. When coadministered with ritonavir/lopinavir combination, bupropion’s area under the curve (AUC) decreased by 57%.6 When combined, a higher dose of up to 100% of the dose may be required.1,6 Also, as the dose of ritonavir increases, the bupropion dose should be proportionately increased.
Vortioxetine and vilazodone have a weak to no inhibitory effect on CYP enzymes and display no drug interactions.6 Literature is scarce with regard to vortioxetine and vilazodone with ritonavir.
Antipsychotics. Aripiprazole is a substrate of CYP3A4 and CYP2D6, and its level can be increased when combined with inhibitors of these enzymes such as ritonavir. When coadministered, aripiprazole should be started at a low dose.1,6 There is sparse literature on the drug interactions between ritonavir and aripiprazole.
Olanzapine is metabolized by CYP1A2 and UGT (UDP-glucuronosyltransferase) 1A4 enzymes. An open-label crossover study8 showed a 53% decrease in the olanzapine AUC when coadministered with ritonavir (300-500 mg twice/day).1,6 If combined with ritonavir-containing antiretroviral therapy, a 50% increase in the dose of olanzapine would be necessary.6
Quetiapine is metabolized by CYP3A4. When quetiapine was coadministered with ritonavir and atazanavir, there was a 50-lb increase in weight and hyperglycemia.6 A case report9 described mental confusion and sedation when quetiapine was combined with the same antiretroviral combination. Another case report10 described a patient who experienced priapism as a result of drug interactions between ritonavir and quetiapine. When a protease inhibitor such as ritonavir is added to a stable dose of quetiapine, the antipsychotic dose should be reduced to one-sixth of its original.6 When quetiapine is added to ritonavir, the lowest possible dose of quetiapine should be used and titrated upward.6
Risperidone is a substrate of CYP2D6 and CYP3A4. Combining ritonavir with risperidone has been shown to increase side effects such as extrapyramidal symptoms, neuroleptic malignant syndrome, altered mental status, and coma.1,6 Angioedema has also be reported due to drug interactions between ritonavir and risperidone.11
Lurasidone is metabolized by CYP3A4.1 It is contraindicated when combined with ritonavir due to an increase in lurasidone concentration.6
Ziprasidone has a high risk of prolonging the QTc interval.1 If coadministered with ritonavir and lopinavir, ziprasidone could potentially have an additive effect and prolong the QTc interval. Such prolonged QTc intervals can lead to life-threatening ventricular arrhythmias, torsades de pointes, and sudden cardiac death.3
Mood stabilizers. Carbamazepine is an inducer of CYP3A4 and CYP1A2, as well as a substrate of CYP1A2. Coadministration with ritonavir has led to an increase in blood levels of carbamazepine to toxic concentrations, causing blood dyscrasia, cardiovascular instability, ocular abnormalities, neurologic deficits, and dermatologic reactions. Carbamazepine should not be coadministered with lopinavir/ritonavir combination due to a potential increase in the concentration of the mood stabilizer.6
Sodium valproate is metabolized by CYP2C9, CYP2B6, and CYP2A6.12 When combined with lopinavir/ritonavir, valproate caused a decrease in the concentration of the mood stabilizer, leading to the development of symptoms of mania and hypomania. However, data on coadministering these drugs are inconclusive, and caution is advised when using the combination.6
Lamotrigine is mainly metabolized via glucuronidation.13 When coadministered with ritonavir/lopinavir combination, its AUC decreases by 50%.6 With this combination, a higher dose of lamotrigine may be necessary.
Lithium is not metabolized and eliminated directly through the kidney.14 There is limited literature regarding its interaction with lopinavir/ritonavir, and the propensity for interactions is very low.
Anxiolytics. Buspirone is a CYP3A4 substrate. In a case report,15 when buspirone was combined with ritonavir-containing antiretroviral therapy, the patient developed parkinsonian symptoms.1,6 Caution is advised when using this combination.
Midazolam is metabolized by CYP3A4. Coadministration of midazolam with ritonavir caused an increase in midazolam concentration.6
CYP3A4 and CYP2C19 metabolize diazepam. When combined with ritonavir, the effects of diazepam may be prolonged. HIV treatment guidelines6 have suggested avoiding this combination. The same holds true for triazolam, which is metabolized through CYP3A4.6
Alprazolam is metabolized by CYP3A4. There is contradicting literature on the coadministration of alprazolam with ritonavir. While 1 case report described no effects, another described sedation and psychomotor retardation.16 Therefore, it is advised to start alprazolam at a low dose and closely monitor the patient.6
If benzodiazepines are indicated, lorazepam, temazepam, or oxazepam should be chosen over alprazolam, as these drugs do not undergo hepatic metabolism.6
Stimulants. Amphetamines are metabolized by oxidation, deamination, and CYP2D6. When combined with ritonavir, an inhibitor of CYP2D6 could potentially increase the levels of amphetamines. In vitro studies17 of methylphenidate indicate that it may inhibit CYP2D6 and CYP2B6. There are sparse data on interactions of stimulants with ritonavir. The clinical significance of these in vitro data is unknown. It is recommended to begin at a low dose when starting these medications.6 Dexmethylphenidate and lisdexamfetamine do not interact with CYP and are considered better options when necessary.6
In summary, these significant drug interactions (Table 1) should be kept in mind before starting psychotropics in a patient on ritonavir/lopinavir combination therapy for COVID-19. When coadministered, the precautions mentioned here should be taken due to the high propensity for interactions.
Received: May 27, 2020.
Published online: June 18, 2020.
Potential conflicts of interest: None.
Funding/support: None.
REFERENCES
1.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. PubMed CrossRef
2.Kim RB. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev. 2002;34(1-2):47-54. PubMed CrossRef
3.Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63(8):769-802. PubMed CrossRef
4.von Moltke LL, Greenblatt DJ, Giancarlo GM, et al. Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab Dispos. 2001;29(8):1102-1109. PubMed
5.Gutierrez MM, Rosenberg J, Abramowitz W. An evaluation of the potential for pharmacokinetic interaction between escitalopram and the cytochrome P450 3A4 inhibitor ritonavir. Clin Ther. 2003;25(4):1200-1210. PubMed CrossRef
6.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services website. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed June 6, 2020.
7.DeSilva KE, Le Flore DB, Marston BJ, et al. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS. 2001;15(10):1281-1285. PubMed CrossRef
8.Penzak SR, Hon YY, Lawhorn WD, et al. Influence of ritonavir on olanzapine pharmacokinetics in healthy volunteers. J Clin Psychopharmacol. 2002;22(4):366-370. PubMed CrossRef
9.Pollack TM, McCoy C, Stead W. Clinically significant adverse events from a drug interaction between quetiapine and atazanavir-ritonavir in two patients. Pharmacotherapy. 2009;29(11):1386-1391. PubMed CrossRef
10.Geraci MJ, McCoy SL, Crum PM, et al. Antipsychotic-induced priapism in an HIV patient: a cytochrome P450-mediated drug interaction. Int J Emerg Med. 2010;3(2):81-84. PubMed CrossRef
11.Gonzalez LS, Kothari K, Kasle DA. Three cases of late onset angioedema in nursing home human immunodeficiency virus patients on ritonavir and risperidone. J Clin Psychopharmacol. 2016;36(1):95-97. PubMed CrossRef
12.Zhu MM, Li HL, Shi LH, et al. The pharmacogenomics of valproic acid. J Hum Genet. 2017;62(12):1009-1014. PubMed CrossRef
13.Fitton A, Goa KL. Lamotrigine: an update of its pharmacology and therapeutic use in epilepsy. Drugs. 1995;50(4):691-713. PubMed CrossRef
14.Wen J, Sawmiller D, Wheeldon B, et al. A review for lithium: pharmacokinetics, drug design, and toxicity. CNS Neurol Disord Drug Targets. 2019;18(10):769-778. PubMed CrossRef
15.Clay PG, Adams MM. Pseudo-Parkinson disease secondary to ritonavir-buspirone interaction. Ann Pharmacother. 2003;37(2):202-205. PubMed CrossRef
16.Greenblatt DJ, von Moltke LL, Daily JP, et al. Extensive impairment of triazolam and alprazolam clearance by short-term low-dose ritonavir: the clinical dilemma of concurrent inhibition and induction. J Clin Psychopharmacol. 1999;19(4):293-296. PubMed CrossRef
17.Markowitz JS, Straughn AB, Patrick KS. Advances in the pharmacotherapy of attention-deficit-hyperactivity disorder: focus on methylphenidate formulations. Pharmacotherapy. 2003;23(10):1281-1299. PubMed CrossRef
aDepartment of Psychiatry, Texas Tech University Health Science Center at Odessa/Permian Basin, Odessa, Texas
bDepartment of Research, De Sousa Research Foundation, Mumbai, India
cDepartment of Psychiatry, Central Michigan University, Saginaw, Michigan
dDepartment of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland
eDepartment of Psychiatry, Penn State College of Medicine, Harrisburg, Pennyslvania
*Corresponding author: Zeeshan Mansuri, MD, MPH, Texas Tech University Health Sciences Center at Odessa/Permian Basin, 2301 W Michigan Ave, Midland, TX 79701 ([email protected]).
Prim Care Companion CNS Disord 2020;22(3):20com02677
To cite: Mansuri Z, Shah B, Adnan M, et al. Ritonavir/lopinavir and its potential interactions with psychiatric medications: a COVID-19 perspective. Prim Care Companion CNS Disord. 2020;22(3):20com02677.
To share: https://doi.org/10.4088/PCC.20com02677
© Copyright 2020 Physicians Postgraduate Press, Inc.
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top